Introduction
Malaria poses a major public health challenge globally. In 2017, there was an estimated 219 million cases of malaria worldwide.1 Eradication therapy in Plasmodium vivax in variants of glucose-6-phosphate dehydrogenase (G6PD) deficiency individuals remains a clinical challenge. We present a case of primaquine-induced haemolysis in a patient with G6PD deficiency (G6PDd) complicated with clinical sequelae of thrombosis.
Case presentation
A 47-year-old Malaysian male, with no known medical illnesses, returned home from Papua New Guinea two months prior to complaints of fever and generalised myalgia which lasted for one week. He had never received malaria chemoprophylaxis before. On examination, he was febrile with a temperature of 38.6ºC and tachycardic with a pulse rate of 110 beats per minute. Other vital sign parameters and physical examinations were unremarkable.
Laboratory investigations showed haemoglobin of 12.7 g/dL, leukocyte of 5,000/μl and platelet of 81,000/μl. Kidney and liver parameters were normal. He was seronegative for human immunodeficiency virus, venereal disease research laboratory test, and hepatitis B and C. His blood film for malaria parasite (BFMP) was reported as P. vivax with presence of 2,400/ul trophozoite and 468/ul gametocytes. Therefore, a diagnosis of uncomplicated P. vivax malaria was made. Artemether/lumefantrine treatment was commenced and completed as per standard regimen. The initial fluorescent G6PD screening test result was normal. The patient was then discharged with daily primaquine Directly Observed Treatment Short-Course (DOTS) which was planned for 14 days as per standard eradication therapy.
Five days after commencing on primaquine, the patient was readmitted for acute ischaemic stroke, manifesting as left-sided hemiparesis with power of 1/5. His Glasgow Coma Scale was full with good orientation. He appeared tachypnoeic and tachycardic. Oxygen saturation (SpO2) under room air measured with a pulse oximeter varied between 70% and 75% and did not increase after application of a non-rebreathing mask with 15 L/min oxygen. He appeared pale, jaundice and cyanotic. Radial artery and venous blood was dark-coloured. Arterial blood gas under room air showed PO2 of 218 mmHg and SO2 of 74 mmHg. There was laboratory evidence of severe haemolysis with haemoglobin of 5.1 g/dL (a drop of 7.6 g/dL compared to baseline), reticulocyte count of 4%, lactate dehydrogenase of 1,268 U/L and predominantly indirect hyperbilirubinemia. Peripheral blood smear was consistent with features of oxidative haemolysis (Figure 1). Coombs test (direct and indirect) was negative. Infective screening including mycoplasma serology and repeat BFMP during this presentation was negative. Repeated fluorescent G6PD screening test remained normal. A computed tomography brain scan showed features of right internal capsule infarct. Due to a resource-restricted setting, the methaemoglobin concentration was unable to be measured by the local laboratory.
Figure 1 Peripheral blood smear showed features of oxidative haemolysis with presence of blister cells, bite cells, polychromasia and occasional microspherocytes (Leishman stain ×400)
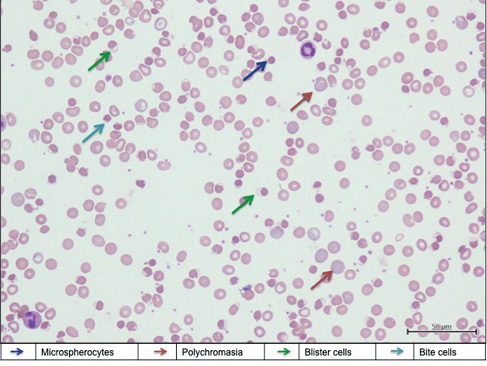
Primaquine-induced methaemoglobinaemia and haemolytic anaemia complicated with acute cerebrovascular accident was suspected due to the oxygen-resistant cyanosis without respiratory compromise or abnormalities on the chest X-ray and the presence of severe haemolysis. Thus, his primaquine treatment was withheld. The patient was started on hyperhydration and forced alkaline diuresis in addition to two pints of packed cell transfusion, steroid, folic acid and intravenous parentrovite, which contained 1,000 mg of ascorbic acid. Oral vitamin C (1,000 mg/day) was also prescribed. The haemolysis resolved with a gradual increment in SpO2 level after nine days of treatment. The patient was discharged with residual left-sided hemiparesis with a power of 2/5.
However, the patient was readmitted after three weeks with complaints of atypical chest pain. Vital signs and physical examinations were unremarkable except the presence of residual left-sided hemiparesis. Electrocardiogram and cardiac markers were not consistent with myocardial infarction. In view of the incomplete eradication therapy for his initial P. vivax infection, a repeat BFMP was performed which showed detection of P. vivax with 480/ul trophozoite and 0/μl gametocyte count. Haemoglobin taken during this admission was 14.1 g/dL. Other parameters did not show any evidence of haemolysis. A repeat G6PD screening test showed evidence of G6PDd and we proceeded to do a quantitative G6PD enzyme activity assay (colorimetric) at a private laboratory. The result was 2.554 unit/g Hb (25.31%), suggesting moderate degree of deficiency.2 A diagnosis of relapse P. vivax malaria infection with clinical sequelae of thrombosis was made. The patient was given a second course of artemether/lumefantrine treatment and was re-challenged with modified regimen of primaquine (0.75 mg/kg) once weekly for eight weeks. The patient was followed up with weekly clinical and laboratory monitoring. He successfully completed the eradication therapy without any adverse reactions. BFMP repeated one month after primaquine completion did not show any evidence of relapse. He was clinically well with residual left hemiparesis with a power of 3/5 and underwent intensive rehabilitation.
Discussion
G6PDd, an X-linked recessive disorder, is the most common enzyme deficiency globally.3 G6PD is expressed in tissues where it catalyses the pentose phosphate pathway reaction in which reduced nicotinamide adenine dinucleotide phosphate (NADPH) is generated. NADPH plays a role in producing reduced glutathione which protects cells against oxidative stress.3 In G6PDd individuals, erythrocytes which lack mitochondria are more susceptible to oxidative damage as they depend solely on G6PD to generate NADPH.3 There are various variants of G6PDd which are classified based on residual enzyme activity and clinical phenotype.4 Common precipitating factors of haemolysis include ingestion of fava beans, infections and drugs that contain primaquine.
The overall prevalence of G6PDd in Malaysia was reported to be 3.4%, with a prevalence among males of 5.3% and among females of 1.1%. G6PD Viangchan and Mahidol are common Southeast Asian variants.5 The G6PD screening test adopted by our laboratory is based on the method of fluorescent spot test. NADPH, which is generated by G6PD and present in a lysate of blood cells, fluoresces under long-wave ultraviolet (UV) light. In G6PDd, there is an inability to produce sufficient NADPH, thus resulting in a lack of fluorescence.6 Erythrocytes with less than 20% of normal G6PD activity do not cause detectable fluorescence in such tests.6 Thus, this screening can miss moderate G6PDd as only erythrocytes with more than 20% of normal G6PD activity will give a fluorescence result, which is interpreted as normal. Our patient had normal G6PD screening prior to primaquine initiation, which suggests that his baseline erythrocytes had higher than 20% of normal G6PD activity. G6PD screening performed when he presented with haemolysis was falsely negative because reticulocytes have higher G6PD activity than mature erythrocytes. This necessitates repeat testing after resolution of haemolysis if there is high clinical suspicion of G6PDd.7 Our patient’s G6PD was found to be deficient three weeks after resolution of haemolysis, which strongly suggests a true G6PDd. A study has shown that primaquine-induced haemolysis in less severe G6PD variants typically starts after two days of exposure, with the average haemoglobin plunging to a nadir five days post-primaquine initiation.8 This is consistent with our clinical finding.
P. vivax malaria has persistent liver stages (hypnozoites) which may result in frequent relapses with intervals as short as three weeks in tropical regions.9 Primaquine is currently the only widely available antimalarial drug for the radical cure of P. vivax infections. Primaquine causes predictable oxidant haemolysis in G6PDd in which the degree of haemolysis depends on the dose administered and the severity of the enzyme deficiency.3 In Malaysia, quantitative G6PD testing is not widely available, and because qualitative assays may lead to falsely normal G6PD level, it is important to have a high clinical suspicion of haemolysis despite normal levels of G6PD in a patient on primaquine with evidence of ongoing haemolysis. Due to limited treatment options in P. vivax eradication, it is difficult to determine the best regimen for patients with G6PDd, and rechallenging with similar drugs may be possible as younger red blood cells are able to withstand oxidative stress better than senescent red blood cells. The current recommended dose for P. vivax malaria eradication is 420 mg (i.e. 30 mg/day for 14 days) primaquine in G6PD normal individuals, whereas the maximum dose administered in patients with G6PDd is 360 mg (45 mg/week for eight weeks). These options were reported to be effective, as evidenced by a relatively low relapse rate.10 No relapse was observed in our patient after completion of eight weekly primaquine treatment.
The mechanism of coagulation activation in haemolytic anaemia is multifactorial, possibly resulting from increased tissue factor activity and endothelial dysfunction.11 Haemolysis-associated nitric oxide depletion, as a result of cell-free oxyhaemoglobin which acts as an nitric oxide scavenger, leads to the production of methaemoglobin.12 Neutrophil extracellular traps are formed via neutrophil activation by free haem, which recruits red blood cells, activate platelets and promotes fibrin deposition. The products of haemolysis generate reactive oxygen species and upregulate pro-inflammatory cytokine responses.13 These effects act together to increase the risk of thrombosis, explaining the thrombotic sequelae observed in our patient.
Vitamin C has been reported to be useful for methaemoglobinaemia.14 Methylene blue is known to precipitate haemolysis in individuals with G6PDd and is potentially hazardous.3 The reason for giving vitamin C to our patient who had clinical evidence of methaemoglobinaemia with cyanosis was based on the high suspicion of G6PDd because the haemolysis onset was in temporal relationship with the timing of primaquine administration, despite the initial normal G6PD results. Our patient had a favourable outcome with vitamin C treatment, as evidenced by the significant improvement in pulse oximeter readings and resolution of haemolysis.
Conclusion
Our case highlighted the challenge posed in managing a patient with P. vivax infection with underlying G6PDd, which unfolded through acute haemolysis episode post-administration of primaquine. Clinicians should be aware that qualitative G6PD tests can be misleading in patients with baseline moderate severity of G6PDd and during acute phase of haemolysis. Clinical suspicion should be heightened in at-risk patients who present with saturation gap and haemolysis in a temporal relationship with any potential triggers. Prompt diagnosis and timely treatment are of utmost importance. 
Acknowledgement
The authors would like to thank the Director General of Health Malaysia for the permission to publish this paper.
Ethical approval
This article was registered with National Medical Research Register of Malaysia (Research ID of NMRR-20-1343-55628).
