Introduction
Rhabdomyolysis is a clinical syndrome characterised by acute damage of the skeletal muscle leading to the release of potentially toxic muscle cell constituents such as creatine kinase (CK) and myoglobin into the systemic circulation. The common causes of rhabdomyolysis include intense exercise, trauma, drugs (e.g. statins), infections, electrolyte disorders (e.g. hypokalaemia, hypophosphataemia) and very rarely, genetic defects in muscle enzymes or channelopathies. This can result in acute kidney injury due to renal tubular blockage by the released myoglobin. Profound hypokalaemia can trigger rhabdomyolysis, which can present with muscle cramps, weakness and fatigability. Rhabdomyolysis due to hypokalaemia is a rare initial presentation of primary hyperaldosteronism (PA). Patients with PA usually present with hypertension and may have biochemical abnormalities such as hypokalaemia secondary to kaliuresis or metabolic alkalosis. The diagnosis is confirmed with evidence of autonomous aldosterone production with suppressed renin concentration or activity.
Case presentation
A 42-year-old Filipino lady presented with progressive proximal upper and lower limb weakness for two weeks. She had difficulty in climbing stairs and standing up from a squatting position. The weakness gradually progressed, resulting in difficulty ambulating on flat ground. She had a history of hypertension which was diagnosed two years earlier. She was on amlodipine 5 mg once daily, without the use of diuretics or over the counter medications.
At presentation, her blood pressure (BP) was 150/75 mmHg, heart rate 88 beats/minute and SpO2 99% on room air. Her height was 160 cm and weight 62 kg, giving her a body mass index of 24.2 kg/m². On neurological exam, proximal weakness (Medical Research Council grading 3/5) was detected in upper and lower limbs bilaterally. There were no features of Cushing’s syndrome. She was clinically euthyroid without a goitre or signs of thyroid eye disease. Systemic examination was unremarkable.
Initial investigations performed at the Emergency Department revealed profound hypokalaemia (potassium 2.1 mmol/L), hypomagnesaemia (magnesium 0.49 mmol/L) and metabolic alkalosis (bicarbonate 35.1 mmol/L). Her CK and urine myoglobin were significantly elevated (Table 1). She was diagnosed with rhabdomyolysis based on the findings of elevated serum CK and urine myoglobin levels, together with proximal muscle weakness. She denied any acute infections, trauma or intoxication. She was not taking any statin or fibrate. There were no signs of autoimmune disease or dermatomyositis.
Table 1 Initial investigations performed on admission
|
|
|
|
|
Urea
|
4.4
|
2.7–6.9 mmol/L
|
|
Sodium
|
140
|
136–146 mmol/L
|
|
Potassium
|
2.1
|
3.6–5.0 mmol/L
|
|
Chloride
|
87
|
100–107 mmol/L
|
|
Bicarbonate
|
35.1
|
19–29 mmol/L
|
|
Glucose
|
5.8
|
3.9–11.0 mmol/L
|
|
Creatinine
|
83
|
37–75 μmol/L
|
|
Magnesium
|
0.49
|
0.74–0.97 mmol/L
|
|
Creatine kinase
|
1,579
|
44–201 U/L
|
|
Urine myoglobin
|
179
|
<25 mcg/L
|
|
FreeT4
|
13
|
8.8–14.4 pmol/L
|
|
Thyroid stimulating hormone
|
1.91
|
0.65–3.70 MU/L
|
Her serum potassium in 2017 was normal (4.1 mmol/L). There was no history of vomiting or diarrhoea to suggest gastrointestinal loss of potassium. She was not given any insulin or beta agonist that could drive potassium intracellularly. Her thyroid function test was normal. There was no family history of periodic paralysis or weakness to suggest familial channelopathy. Her chest X-ray was normal without signs of cardiomegaly or heart failure. Electrocardiogram showed normal sinus rhythm with prolonged QTc of 473 milliseconds, although no U waves were seen.
She was started on intravenous potassium chloride infusion together with oral potassium supplements (Span-K 1.8 g three times daily). She was also given intravenous magnesium sulphate to correct hypomagnesaemia as magnesium deficiency can exacerbate potassium wasting and render treatment of hypokalaemia refractory. Her rhabdomyolysis also ameliorated with intravenous hydration. She regained full strength of both upper and lower limbs after normalisation of serum potassium. Her spot urine potassium was raised at 41 mmol/L (corresponding serum potassium of 2.4 mmol/L), suggesting renal wasting of potassium. Her 24-hour urine potassium was also significantly elevated at 86 (10–44 mmol/day). Hypokalaemia with renal potassium wasting, metabolic alkalosis and history of hypertension suggest the possibility of mineralocorticoid excess, and hence screening for PA was carried out.
Her plasma aldosterone concentration (PAC) was 424.6 (97.3–834 pmol/L) and direct renin concentration was 1.3 (upright: 1.8–59.4 ng/mL, supine: 1.1–20.2 ng/mL), with an aldosterone renin ratio (ARR) of 11.8. The corresponding serum potassium was 3.3 mmol/L. ARR of greater than or equal to 3.8 is suggestive of a positive case detection for PA.1 ARR is expected to be higher if it would have been sent off in a potassium replete state as hypokalaemia is known to suppress aldosterone secretion.1
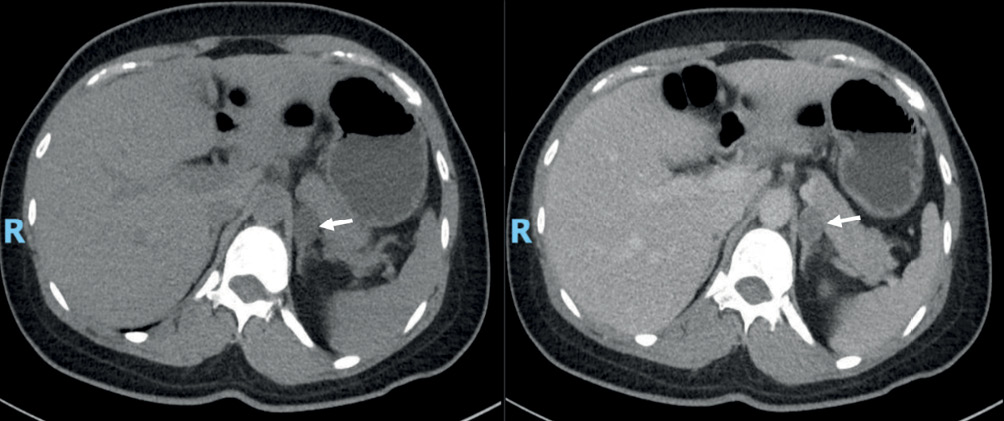
Intravenous salt loading test was done to confirm PA. She was given 2 L of 0.9% normal saline over four hours before a PAC measurement was performed. Post-saline infusion PAC of 898 pmol/L in the patient was consistent with a diagnosis of PA.1 She was keen for surgical treatment if excess aldosterone was localised to a unilateral source. Computed tomography of adrenal glands revealed a 1.8 × 1.5 cm hypodense lipid rich adrenal nodule (white arrows) with attenuation density of 5 HU on an unenhanced scan of the left adrenal gland (Figure 1).
Figure 1 CT of adrenal gland showing left adrenal nodule (arrows), before (left) and after contrast administration (right)
Adrenal vein sampling was organised to assess the source of excess aldosterone. This confirmed autonomous aldosterone production from the left adrenal gland. She underwent laparoscopic left adrenalectomy with normalisation of BP and potassium postoperatively. The cut section of the left adrenal gland showed two discrete nodules (Figure 2). Both nodules showed similar appearance and were well-circumscribed, solid and yellowish without any haemorrhage or necrosis. The histology of the resected specimen was consistent with adrenocortical adenoma.
Figure 2 Macroscopic appearance of two left adrenal nodules showing well-circumscribed and encapsulated with a typical golden yellow appearance
Discussion
Rhabdomyolysis presenting with severe hypokalaemia as the first manifestation of PA is extremely rare. Severe muscle weakness or rhabdomyolysis usually occurs only if serum potassium is below 2.5 mmol/L To the best of our knowledge, there are 16 cases of rhabdomyolysis caused by hypokalaemia from PA reported in the literature thus far.2 Hypokalaemia is present in only 9–37% of patients with PA.1 PA associated with hypokalaemia-induced rhabdomyolysis is more common in Asians, as seen in our patient.3
Multiple epidemiological studies have shown that PA has a prevalence of >5% (possibly even >10%) in hypertensive patients, both in general and specialty settings.4 PA occurs in ~5% of the adult hypertensive patients in Singapore.5 The prevalence of PA is increased in relation to the severity of the hypertension.6 PA was present in only 2% of patients with mild hypertension (<160/100 mmHg) in a study by Mosso et al.7 It is interesting that our patient presented with severe biochemical manifestations of PA despite satisfactory BP control on amlodipine. There is a possibility that suppression of the endogenous renin–angiotensin–aldosterone system and vasodilatory effects of oestrogens and progesterone on the vascular endothelium in females may result in either mild hypertension or normotension, despite hyperaldosteronism in PA patients.8
PA is a multisystem disease where aldosterone excess directly or indirectly affects several organs. Besides its role in the regulation of extracellular volume and BP, mineralocorticoids also exert multiple biological actions in other tissues. Patients with PA are at higher risk of developing long-term cardiovascular and cerebrovascular events independent of age, gender and BP.9 In a meta-analysis of 31 studies, patients with PA were at increased risk of cardiovascular complications such as stroke, coronary artery disease, atrial fibrillation, left ventricular hypertrophy and heart failure.10 Moreover, PA also significantly increased the risk of atherogenic conditions such as diabetes mellitus and metabolic syndrome.10
Given our patient’s young age, and the presence of unilateral aldosterone-producing adenoma (APA), laparoscopic adrenalectomy would be the treatment of choice as it offers the possibility of a cure. It is the preferred surgical approach and is associated with a shorter recovery time, decreased length of stay and fewer postoperative complications compared to open approach.1 Alternatively, medical therapy would involve a lifetime of medications and monitoring.
Recent studies have shown that adrenalectomy is superior to medical therapy in relation to cardiovascular and renal outcomes. In a large prospective study, medically treated PA patients had a higher incidence of atrial fibrillation compared with adrenalectomised PA patients during the 12-year follow-up period.11 Another study showed that arterial stiffness was reduced following adrenalectomy, but not after one year of spironolactone treatment for PA.12 Patients with unilateral APA had reduced progression to end-stage renal failure and lower mortality than hypertensive controls after adrenalectomy. Mineralocorticoid receptor antagonist did not significantly alter those outcomes in a population database study of PA patients from Taiwan.13 In relation to the resolution of hypertension, surgical but not medical treatment was significantly associated with amelioration of hypertension among those with APA in a Japanese epidemiological study.14 Female gender, fewer than or equal to two anti-hypertensive medications, duration of hypertension less than or equal to six years and a body mass index less than or equal to 25 kg/m² were identified as the best predictors of complete resolution of hypertension post-adrenalectomy.15 Our patient fulfilled all this criteria and she achieved normalisation of BP after adrenalectomy.
Medical treatment, including a mineralocorticoid receptor antagonist, should only be recommended if a patient is unwilling to undergo surgery or has bilateral PA. Spironolactone is generally considered first-line therapy. However, if a patient is unable to tolerate this, treatment can be with eplerenone, a selective mineralocorticoid receptor blocker with fewer antiandrogenic effects.1
Conclusion
Although it is extremely rare, rhabdomyolysis from hypokalaemia can be the first presentation of PA. Screening for PA should be done in any patient with hypertension and hypokalaemia, regardless of the severity of hypertension and BP control. In patients with unilateral PA, the benefits of adrenalectomy clearly outweigh medical therapy in relation to amelioration of hypertension and hypokalaemia, and in long-term cardiovascular and renal outcomes.
