Introduction
Mucormycosis is a serious but rare fungal infection caused by mucormycetes (mold). Infection occurs by encountering the fungal spores from the environment. It usually occurs in individuals who have comorbid conditions or immunocompromised conditions. The fungus can enter the skin through cuts, burns, or different type of trauma to the skin. The fungal hyphae invade the blood vessels, causing tissue thrombosis and necrosis in a rapid progression (Figures 1 and 2).1-3
Figure 1 Shows a patient with right-sided orbital swelling , blackening of right sided face and nose with loss of vision in the right eye, patient had no history of previous COVIDinfection but was a known to have chronic liver disease and alcoholism.
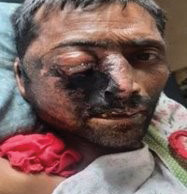
Figure 2 Shows a patient with bilateral orbital swelling with loss of vision, with swelling and tenderness of the bilateral maxillary area and swelling around the forehead, possible fungal extension, diagnosed as ROCM, newly diagnosed diabetes mellitus, with a post-COVID status.
It is rare, affecting around 1.7 people per million population each year. It is more prevalent in India (0.14 cases per 1,000 population), and its incidence is increasing at a greater rate.4 Increased incidence of mucor was reported in India after the second wave of COVID from January 2021 onwards. The sudden surge in cases was unexpected but in many individuals, the cause could easily be explained by diabetes, immuno-suppression with contribution from the COVID virus itself. Simultaneously there were many cases without any obvious pre-disposing factors. Due to the unexpected rise in COVID cases in late January 2021, India registered an increased use of evidence-based, experimental and a few unproven interventions by patients themselves and sometimes by treating physicians to counter this pandemic or the fear associated with it.
The clinical course of COVID were complicated by superadded infections which are more harmful to the patients. The most common superadded infections were bacterial, but the emergence of fungal infections was of major concern. In the early part of the COVID pandemic, less than 1% of secondary fungal infections were recorded.5,6 Comorbid conditions, glucocorticoids and the use of antibiotics were greater than desired clinical dosing, and a lapse in infection control were factors leading to secondary mucormycosis in COVID patients.7 Awareness increased among treating physicians and microbiologists after an abrupt rise in the incidence of fungal infection during the second wave of COVID.8,9 An analysis done in hospitalised COVID patients reported ≈6% fungal infections.10 An increased incidence of mucor in India was reported from different cities during the second wave of the COVID pandemic. A city in South India reported around 18 cases simultaneously, which raised our concerns about mucormycosis in post-COVID patients.11-13 Mucormycosis is mainly associated with the immunocompromised status of patients; the most common comorbidity was diabetes but COVID in India provided the necessary ingredient, as India has greater incidence of mucor, this combination of COVID with diabetes led to an abrupt surge of mucor in the country.12,14
This analysis was conducted with the objective of defining the association between COVID and various other factors such as uncontrolled diabetes, the use of more than desired clinical dosing of antibiotics and steroids, and other immune-compromised status. COVID was major part of this analysis.
Methods
Study design
This was an observational study to appraise the various factors which resulted in an abrupt surge of mucormycosis during the COVID-19 pandemic (second wave) in India.
The present analysis was done at a tertiary level post graduate institute in central India, where a separate multidisciplinary team was constituted for treatment of this disease. All adult patients presenting with clinical history, symptoms and signs suggestive of mucor with radiologically and/or microbiologically confirmed cases admitted from 8/5/2021 to 8/7/2021 to clinical departments of our hospital were included in our analysis after taking an informed consent from the patients/attendants.
Participants
Adults presenting with symptoms and signs of facial swelling (unilateral/bilateral), headache, fever, black lesion on the face, nasal bridge or inside the mouth that quickly becomes more severe, sinus congestion, shortness of breath, cough, abdominal pain, delirium, coma were considered. Clinical cases were confirmed by nasal endoscopy findings, KOH stain, histopathology findings and radiological investigations.
Mucormycosis was diagnosed based on clinical findings, KOH stain and histopathology findings showing non-septate hyphae, which are ribbon like and broad based with wide-angle branching and radiological investigations. COVID-19 was confirmed in patients by RT-PCR or rapid antigen test (RAT) from samples taken from the nasopharynx. Individuals presenting with mucormycosis directly without any previous history of COVID were also included in our study.
Assessments
We used a structured proforma to collect the following details: demographic data along with COVID-19 history, severity, duration of symptoms, different sites of involvement of mucor, hospitalisation (ICU/ward stay) or advised home isolation history during COVID was also recorded. We investigated the treatment offered to patients. We asked about the history of antibiotics use, glucocorticoids, antiviral drugs, biologicals, and other alternative treatments for COVID. Participants were asked about oxygen mask use, canula, cylinders and humidifiers. A history of comorbidities was sought. Investigations included nasal swab KOH mount, CD4 cell count, HIV antibody test, COVID antibody test in individuals who did not have a history of COVID-19 and who were COVID antigen negative, HbA1c, diagnostic nasal endoscopy (DNE) and biopsy, radiological investigations, and outcome in respect to mortality. All this information was noted and analysed.
Statistical analysis
Data were entered into Microsoft excel spreadsheets and studied using open source software. Continuous data were expressed as mean and standard deviations (SD). Categorical data were expressed as percentages.
Ethical approval
The analysis protocol was reviewed, and ethical approval was granted by the Ethics Committee of the institution (No. EC/MGM/AUG-21/21, Dated 21 August). Written informed consent was obtained from the patient/guardian for publication of this paper (including images, case history and data).
Results
In our analysis of 464 patients, one patient was younger than 20 years, 28 (6%) were 21-30 years, 91 (20%) were 31-40 years, 163 (35%) were 41-50 years, 88 (19%) were 51-60 years and 93 (20%) were older than 60 years. 316 (79%) were male and 148 (21%) were female (Table 1).
Table 1 Age and gender distribution
|
|
|
|
<=20
|
1
|
|
21-30
|
28 (6%)
|
|
31-40
|
91 (20%)
|
|
41-50
|
163 (35%)
|
|
51-60
|
88 (19%)
|
|
>60
|
93 (20%)
|
|
|
|
|
Male
|
316 (79%)
|
|
Female
|
148 (21%)
|
Out of 464 patients, 287 (62%) were post COVID, 15 (3%) were COVID antigen negative, and 162 (35%) did not have a history of COVID (of which 120 were antibody positive). 29 (6%) were vaccinated and 435 (94%) were non-vaccinated. 123 (27%) patients had less than 10 days duration between COVID and mucormycosis, 79 (17%) patients had 10-20 days duration between COVID and mucor, and 85 (18%) patients had more than 20 days duration between COVID and mucormycosis, and 162 (35%) patients did not have a history of COVID-19. Out of a total of 464 patients, 66 (14%) were hospitalised in the ICU, and 135 (29%) were hospitalised in a ward for COVID previously, while 101 (22%) were treated at home (home isolation), and 162 (35%) did not receive any treatment (Table 2).
Table 2 History of COVID status, COVID vaccination status, duration between COVID-19 and mucor symptoms, and hospitalisation status (COVID)
|
|
|
|
|
|
Post COVID
|
287 (62%)
|
|
COVID negative
|
15 (3%) (antibody positive)
|
|
No history of COVID
|
162 (35%) (120 antibody positive)
|
|
Total
|
464
|
|
|
|
|
|
|
Not vaccinated
|
435 (94%)
|
|
Vaccinated
|
29 (6%)
|
|
Total
|
464
|
|
|
|
|
|
|
COVID negative
|
15 (3%)
|
|
<10 days
|
123 (27%)
|
|
10-20 days
|
79 (17%)
|
|
>20 days
|
85 (18%)
|
|
No history of COVID
|
162 (35%)
|
|
Total
|
464
|
|
|
|
|
|
|
Hospitalisation
|
66 (14%) ICU admission
135 (29%) ward admission
|
|
Home isolation
|
101 (22%)
|
|
No treatment
|
162 (35%)
|
|
Total
|
464
|
163 (35%) out of 464 admissions had less than or equal to seven days history of glucocorticoid use during COVID, 94 (20%) had more than seven days history of use, 207 (45%) did not have a history of steroid intake. 280 (60%) (majority) individuals managed with antibiotics during COVID and 97 (21%) with remdesivir, 57 (12%) with favipiravir, 74 (16%) with zinc tablets, 10 (2%) with biologicals, 12 (2.5%) with plasma/IVIG, and 162 (34%) patients did not have a history of treatment. Out of the 464 patients, 417 (90%) did not have a history of mask reuse and 47 (10%) did have a history of mask reuse. 32 (7%) individuals had a history of treatment with alternative medicine during COVID (Table 3).
Table 3 History of steroid use, drug use, mask reuse, use of alternative medicine
| |
|
|
|
|
|
|
Yes
|
≤7
|
163 (35%)
|
|
|
>7
|
94 (20%)
|
|
No
|
|
207 (45%)
|
|
Total
|
|
464
|
|
|
|
|
|
|
Antibiotics
|
280 (60%)
|
|
Zinc
|
74 (16%)
|
|
Remdesivir
|
97 (21%)
|
|
Febiflu
|
57 (12%)
|
|
Biologicals
|
10 (2%)
|
|
IVIG/plasma
|
12 (2.5%)
|
|
No history of any treatment
|
162 (35%)
|
|
Total
|
464
|
|
|
|
|
|
|
Yes
|
47 (10%)
|
|
No
|
417 (90%)
|
|
Total
|
464
|
|
|
|
|
|
|
Yes
|
32 (7%)
|
|
No
|
432 (93%)
|
|
Total
|
464
|
Out of 287 post-COVID participants, 61% were known diabetics, 19% were newly diagnosed and 20% were non-diabetic. Out of 162 patients who did not have a history of COVID, 72 (44%) were known diabetics, 58 (35%) were newly diagnosed, and 32 (20%) were non-diabetic. There were 15 COVID-negative individuals, of which 8 (53%) were previously diagnosed as diabetic, 3 (20%) were newly diagnosed, and 4 (27%) were non diabetic. Of 287 post-COVID participants, 125 (44%) had RM, 102 (35%) had ROM, and 60 (21%) had ROCM. Of 15 COVID-negative individuals, 5 (33%) had RM, 6 (40%) had ROM, and 4 (27%) had ROCM. Of 162 patients who did not have a history of COVID, 93 (57%) had RM, 48 (30%) had ROM, and 21 (13%) patients had ROCM. Of the 464 patients, HIV tests performed on 94 patients were negative, a CD4 count was done in 94 patients, and 49 (52%) had a CD4 count between 500-1000, 35 (38%) had a CD4 count between 200-500, 5 (5%) had a CD4 count less than 200 and 5 (5%) individuals had a CD4 count greater than 1000 (Table 4).
Table 4 Clinical examination including diabetes status, anatomic site of involvement and CD4 count
| |
|
| |
|
|
|
|
Known diabetic
|
175 (61%)
|
8 (53%)
|
72 (44%)
|
|
Newly diagnosed diabetic
|
54 (19%)
|
3 (20%)
|
58 (35%)
|
|
Non-diabetic
|
58 (20%)
|
4 (27%)
|
32 (20%)
|
|
Total patients
|
287
|
15
|
162
|
| |
|
|
|
|
|
|
|
Rhino-mucormycosis
|
125 (44%)
|
5 (33%)
|
93 (57%)
|
|
Rhino-oculo-mucormycosis
|
102 (35%)
|
6 (40%)
|
48 (30%)
|
|
Rhino-oculo-cerebral-mucormycosis
|
60 (21%)
|
4 (27%)
|
21 (13%)
|
|
Total patients
|
287
|
15
|
162
|
| |
|
| |
|
|
|
<200
|
5 (5%)
|
Negative
|
|
200-500
|
35 (38%)
|
Negative
|
|
500-1000
|
49 (52%)
|
Negative
|
|
>1000
|
5 (5%)
|
Negative
|
|
Total
|
94
|
Negativ
|
In 162 patients, the KOH mount was positive (Figure 3), and 83 were histopathology positive. A total of 464 patients were admitted, 60 died, out of which the majority (48 [80%]) were ROCM and 12 (20%) were ROM. No deaths occurred in patients with RM (Table 5). Maximum mortality occurred at age older than 50 years (37 [62%]), 41-50 years mortality was 9 (15%), 31-40 years mortality was 12 (20%), 21-30 years mortality was 2 (3%), no mortality occurred younger than 20 years (Table 6). 15% mortality was reported in 374 diabetic patients, 16% mortality was reported in 257 admissions with a history of glucocorticoids use, and 25% mortality was reported in 241 ICU admissions (Table 7).
Figure 3 KOH stain and histopathology positive patients
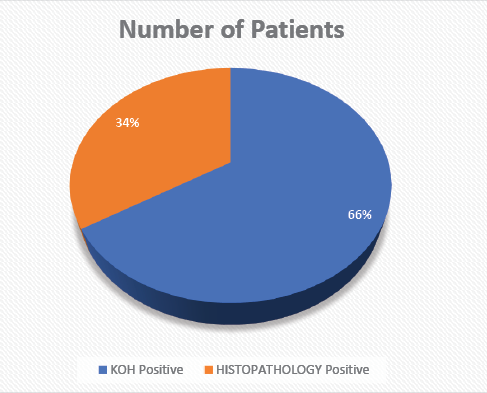
Table 5 Anatomic site involvement and mortality association
|
|
|
|
|
Rhino-mucocormycosis
|
223 (48%)
|
0
|
|
Rhino-oculo-mucocormycosis
|
156 (33.6%)
|
12 (20%)
|
|
Rhino-oculo-cerebral-mucocormycosis
|
85 (18.4%)
|
48 (80%)
|
|
Total
|
464
|
60 (13%)
|
Table 6 Age distribution of mortality
|
|
|
|
<=20
|
0
|
|
21-30
|
2 (3%)
|
|
31-40
|
12 (20%)
|
|
41-50
|
9 (15%)
|
|
>50
|
37 (62%)
|
Table 7 Mortality associated with diabetes, steroid use, ICU admissons
|
|
|
|
|
Known diabetic
|
255
115
|
38 (15%)
19 (16%)
|
|
Newly diagnosed diabetic
|
|
Total diabetics
|
370
|
57 (15%)
|
|
Steroid usage
|
257
|
41 (16%)
|
|
ICU admission
|
241
|
60 (25%)
|
Discussion
The incidence of mucormycosis increased from May 2021 in India. We credit this increase mostly to the COVID-19 pandemic (second wave). The majority of cases had less than 10 days’ gap between COVID and mucormycosis. The inappropriate dosing of glucocorticoids and antibiotics were mainly linked to this epidemic. During the COVID pandemic (second wave), glucocorticoids and antibiotics were used in greater quantity than the desired clinical dosing. The death rate was around 60 (13%) out of 464 admissions, which was less compared to other centres.11 Advanced age (older than 50 years), ICU admissions, and involvement of the brain in mucormycosis has been linked to higher mortality. The use of antifungals is mainly linked to an improved survival rate, since its proactive adequate use to treat mucormycosis.
In our analysis, 316 (79%) patients affected by mucormycosis were men, as seen in previous studies.15-17 We observed diabetes as one of the important underlying comorbidities in both COVID-associated mucor and in individuals who did not have a history of COVID. SARS-CoV-2 mainly affects insulin producing cells (B-cells) of the pancreas, which causes diabetes.18,19 The COVID antibody test was positive in individuals who did not have a history of COVID, and who had negative COVID antigens but had signs and symptoms of COVID. HbA1c levels were also measured in all diabetic participants, which predicted that many individuals had uncontrolled diabetes. Out of 287 post-COVID patients, 61% (majority) were known diabetics, 19% were newly diagnosed and 20% were non-diabetic. Out of the 162 patients who did not have a history of COVID, 44% were known to be diabetic, 35% were newly diagnosed, and 20% were non-diabetic. Of 15 COVID-negative patients, 53% were known to be diabetic, 20% were newly diagnosed, and 27% were non-diabetic.
We believe that glucocorticoids use could be linked to mucor as it down regulates macrophages and neutrophils, causing further deterioration of the immune system in fighting infection, which increases the likelihood of secondary infections. Glucocorticoids causes high blood sugar, which provides an acidic medium for mucormycosis. Around 435 (94%) of the patients were not vaccinated with covishield or covaxin (COVID vaccines), which could be the reason for the mucor infection. It is possible that COVID causes immune dysregulation, ultimately predisposing individuals to mucor, however, this is unproven.20-22 During the recovery phase from COVID infection, there is a persistence of immune dysregulation which may be linked to fungal infection. Use of biologicals in the treatment of COVID has been suspected as a linking factor for fungal infection.23 Only 10 (2%) of individuals received biologicals during COVID out of the 464 admissions.
Our study suggests advanced age as a major factor related to hospitalisation, respiratory system failure, admissions in ICU, and glucocorticoid use in COVID.24,25 Being older than 50 years was major factor related to mortality in our institute. We did not observe many cases of pulmonary and gastrointestinal mucor. As there are minimum cases showing involvement of respiratory and gastrointestinal systems, we think it could be due to the complications of COVID or treatment related to COVID, rather than a sole modification in the respiratory or gastrointestinal systems. Several pulmonary or gastrointestinal mucor cases have not been diagnosed because it is difficult to obtain respiratory or gastric samples in critical patients admitted to ICU.
The treatment involves antifungal therapy to be started as soon as possible and surgery of the infected part, where feasible. The major drawbacks in the treatment of mucor were the financial conditions of the individuals and the gap in treatment protocol. They could not afford costly antifungals (liposomal amp-B, posaconazole). Data suggest a lower mortality rate in patients of mucor when treated with a combination of both antifungals and surgery (19-44%), when compared with only antifungal therapy (50-61%).27 Posaconazole was mainly used when we did not see a favourable response after giving Amp-B.28 An analysis from South India of the safety and efficacy assessment of posaconazole in an ROCM study showed no mortality; around 66.6% were completely cured, and the rest of the individuals showed improvement in their condition.29 The new antifungal isavuconazole has shown similar effectiveness to Amp-B. It is recent to the Indian market and its effectiveness still requires assessment.30 The overall death rate due to mucor is 28-52% in India,31 and the death rate specifically for ROCM is 31-49%, but in our analysis the death rate for ROCM was 48 out of a total 464 patients (10%).32,33
Timely and appropriate dosing of antifungals and surgery, where feasible, are essential in the treatment of mucor. Out of 464 patients admitted, around 241 (52%) patients received an injection of AMP-B, and around 156 (33.6%) were treated with intravitreal AMP-B. Around 223 (48%) patients received posaconazole. The evidence is not very clear about the advantages and disadvantages of combining antifungals for mucormycosis treatment.25 Early and aggressive surgery along with antifungals was linked to better survival in the ROCM group in our study, and previous studies also revealed the same outcome.26 The optimal dose and duration of antifungals are not clear in mucormycosis management. An RCT could be done to assess the results of combining antifungals in mucormycosis management. Around 7% of mucormycosis patients were managed with alternative medicine during their COVID infection.
Our study has certain limitations, the first being we analysed the evidence collected from a single centre. The major factor related to mucor in our study was diabetes, which is similar to other countries such as Mexico, China, Iran, Bangladesh, and Pakistan which also had more cases of mucor due to diabetes.34 Further analysis is required to compare evidence of more cases of diabetes and mucor from different countries, particularly from the USA and Europe, where mucor is mainly seen in malignancies, especially hematological and after organ transplantation.35 Contamination from ventilators, air conditioning units, and construction works has caused mucor in the past.36 The burden of spores of mucormycetes could not be estimated in our institute.37 Lastly, we have limited evidence on the timing of the use of antifungals, surgery, or duration/dose of sequential antifungals, which can be factors related to outcome in mucormycosis. Factors such as genetic predisposition could explain why there is high occurrence of mucor infection in India. Thus, analysis of the epidemiology of mucor associated with COVID from countries other than India, i.e. those countries affected by mucor during COVID-19, is urgently needed.
In conclusion, our study has several points of interest regarding mucormycosis in relation to COVID. Advanced age (over 50 years) is related to a higher risk for mortality – 48 (80%) out of total mortality (60) reported in ROCM. 25% mortality reported out of 241 ICU admissions. One hundred and sixty two patients did not have a history of COVID infection, but they were COVID antibody positive, underscoring the fact that there must be some underlying exposure to the COVID antigen. Around 80% patient had dysglycemia, which suggests diabetes as an independent factor related to mucor, possibly because chronic hyperglycemia can compromise the immunity. The COVID pandemic (second wave) leading to mucor in India, glucocorticoids and antibiotics used in greater quantity than the desired clinical dosing, could be other factors, and a debate about unhygienic delivery of oxygen to individuals with COVID are presumed to be potential causes behind the sudden surge of mucormycosis. Industrial oxygen use is presumed to be one factor related to the abrupt surge in this disease, because there is a dissimilarity in the manufacturing of both industrial and oxygen used for medical purposes. Due to a shortage of medical oxygen during the COVID pandemic, doctors used industrial oxygen. However, most individuals with mucor without a known history of infection leaves a gap and the need for further analysis of complex immunopathological studies of patients with COVID prone to mucor, suggesting a direct alteration in cellular immunity due to novel COVID which needs further appraisal. 
Acknowledgement
The authors would like to thank the teaching faculty and colleagues in the Department of Medicine, M.G.M. Medical College & M.Y. Hospital, Indore (MP), India.
References
1 Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect 2009; 15: 2-9.
2 Petrikkos G, Skiada A, Lortholary O et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012; 54: S23-34.
3 Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000; 13: 236-301.
4 Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update”. J Fungi (Basel) 2020; 6: 265.
5 Baiou A, Elbuzidi AA, Bakdach D et al. Clinical characteristics and risk factors for the isolation of multi-drug-resistant Gram-negative bacteria from critically ill patients with COVID-19. J Hosp Infect 2021; 110:165-71.
6 Ripa M, Galli L, Poli A et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect 2021; 27: 451-57.
7 Seaton RA, Gibbons CL, Cooper L et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect 2020; 81: 952-60.
8 Nucci M, Barreiros G, Guimarães LF et al. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021; 64: 152-56.
9 van Arkel ALE, Rijpstra TA, Belderbos HNA et al. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med 2020; 202: 132-35.
10 Chong WH, Saha BK, Ananthakrishnan Ramani et al. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021; 49: 591-605.
11 Garg D, Muthu V, Sehgal IS et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia 2021; 186: 289-98.
12 Ahmadikia K, Hashemi SJ, Khodavaisy S et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 2021; 64: 798-808.
13 Moorthy A, Gaikwad R, Krishna S et al. SARS-CoV-2, uncontrolled diabetes, and corticosteroids – an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg 2021; 20: 1-8.
14 Joshi SR, Das AK, Vijay VJ et al. Challenges in diabetes care in India: sheer numbers, lack of awareness and inadequate control. J Assoc Physicians India 2008; 56: 443-50.
15 Patel A, Kaur H, Xess I et al. A multicentre observational study on the epidemiology, risk factors, management, and outcomes of mucormycosis in India. Clin Microbiol Infect 2020; 26: 944. e9-15.
16 Jeong W, Keighley C, Wolfe R et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 2019; 25: 26-34.
17 Prakash H, Ghosh AK, Rudramurthy SM et al. A prospective multicentre study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol 2019; 57: 395-402.
18 Müller JA, Groß R, Conzelmann C et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab 2021; 3: 149-65.
19 Accili D. Can COVID-19 cause diabetes? Nat Metab 2021; 3: 123-5.
20 Files JK, Boppana S, Perez MD et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest 2021; 131: e140491.
21 Potenza L, Vallerini D, Barozzi P et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood 2011; 118: 5416-19.
22 Ghuman H, Voelz K. Innate and adaptive immunity to Mucorales. J Fungi (Basel) 2017; 3: 48.
23 Cornely OA, Alastruey-Izquierdo A, Arenz D et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019; 19: e405-21.
24 Levin AT, Hanage WP, Owusu-Boaitey N et al. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol 2020; 35: 1123-38.
25 Pijls BG, Jolani S, Atherley A et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open 2021; 11: e044640.
26 Muthu V, Agarwal R, Dhooria S et al. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis. Clin Microbiol Infect 2021; 27: 538-49.
27 Jeong W, Keighley C, Wolfe R et al. Contemporary management and clinical outcomes of mucormycosis: a systematic review and meta-analysis of case reports. Int J Antimicrob Agents 2019; 53: 589-97.
28 Cornely OA, Alastruey-Izquierdo A, Arenz D et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019; 19: e405-21.
29 Manesh A, John AO, Mathew B et al. Posaconazole: an emerging therapeutic option for invasive rhino-orbito-cerebral mucormycosis. Mycoses 2016; 59: 765-72.
30 Marty FM, Ostrosky-Zeichner L, Cornely OA et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016; 16: 828-37.
31 Prakash H, Ghosh AK, Rudramurthy SM et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol 2019; 57: 395-402.
32 Roden MM, Zaoutis TE, Buchanan WL et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41: 634-53.
33 Jeong W, Keighley C, Wolfe R et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 2019; 25: 26-34.
34 Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel) 2019; 5: 26.
35 Rammaert B, Lanternier F, Zahar JR et al. Healthcare-associated mucormycosis. Clin Infect Dis 2012; 54: S44-54.
36 Walther G, Wagner L, Kurzai O. Outbreaks of mucorales and the species involved. Mycopathologia 2020; 185: 765-81.
37 Prakash H, Singh S, Rudramurthy SM et al. An aero mycological analysis of Mucormycetes in indoor and outdoor environments of northern India. Med Mycol 2020; 58:118-23.
