Introduction
Shortness of breath in a postoperative patient might be due to several causes. However, this delayed presentation of a subarachnoid-pleural fistula (SPF) causing significant pleural effusion is quite uncommon, especially following repair of the dural breach, and is an extremely rare cause of pleural effusion. Given the expansion of surgical liaison services, it is important for physicians to be mindful of this complication.
Case presentation
The patient was a 72-year-old female who presented with a five-day history of worsening acute on chronic back pain causing bilateral lower limb weakness and numbness. She had previously been under the care of chronic pain specialists; however, she had ceased to take analgesics 20 years ago due to perceived cognitive side effects. She had not visited a doctor regarding her back pain for 12 years prior to admission and her only regular medication was atorvastatin and bezafibrate for familial hyperlipidaemia. She was previously employed as a nurse but retired on medical grounds in 1991 due to increasing back pain and disability. Prior to her admission, she lived with her husband in a bungalow and was mobile using a walking stick.
Computed tomography of her spine confirmed extensive calcification of the prolapsed discs, and magnetic resonance imaging (MRI) of her spine demonstrated a giant left paracentral disc protrusion at T9–T10 level causing very severe cord compression at this level.
Transthoracic discectomy was performed with the 9th rib partially resected. The T9–T10 disc was strongly adherent to the dura and a small dural tear was caused following removal of a giant intradural fragment at the end of the discectomy. The incidental dural tear was repaired intraoperatively with suture and sealant, and a non-suction chest drain with a water seal chamber was inserted, which initially drained at a rate of approximately 400 mL of serosanguinous fluid per day increasing to 500 mL drained over an 11-hour period on the fourth postoperative day. The patient reported headache and nausea consistent with cerebral hypotension. Fluid from the chest drain tested positive for β2-transferrin, a sensitive marker of cerebrospinal fluid (CSF). Her MRI thoracic spine demonstrated a paraspinal fluid collection which communicated with the pleural cavity and the anterior epidural space via the left T9–T10 exit foramina (Figure 1) consistent with SPF.
Figure 1 Axial T2 image of MRI spine demonstrating likely SPF at T9–T10 level marked with an arrow
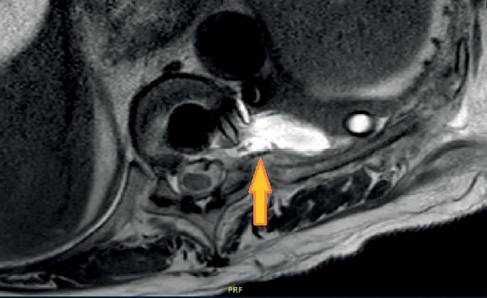
Figure 2 Chest X-ray demonstrating moderate left-sided pleural effusion marked with an arrow
Her symptoms gradually improved with bed rest and she was stepped down from the High Dependency Unit (HDU) on day 9 postoperatively, once the chest drain was removed, to the spinal-orthogeriatric unit where she was regularly reviewed by elderly care physicians as part of their spinal liaison role.
Although she remained stable initially, by week 4 postoperatively, she started feeling dyspnoeic as well as dizzy and sweaty and she had mild headaches. Clinical examination revealed decreased air entry in the left chest posteriorly from the mid to lower zone with dullness to percussion suggestive of left-sided pleural effusion. She did not have any neck stiffness but reported mild photophobia. She was apyrexial, normal C-reactive protein and there was no evidence of leukocytosis in her blood test. Her chest X-ray (Figure 2) showed moderate left-sided pleural effusion.
Following geriatrician review, an urgent respiratory opinion was sought, and she underwent an ultrasound-guided diagnostic tap. The pleural aspirate revealed no growth. Given her symptoms of photophobia and headache, a pleural fluid sample was sent for β2-transferrin, which tested positive and thus confirming recurrent SPF.
She was kept on flat bed-rest for a week, with periods of head-down tilt every four hours as tolerated. Microscopy and culture of the pleural fluid did not demonstrate any evidence of infection and, following discussion with the microbiology department, she was not started on any prophylactic antibiotics.
Serial chest X-ray demonstrated resolving pleural effusion and the patient reported improvement in her symptoms with conservative management. With ongoing physiotherapy and rehabilitation, she started mobilising with a Zimmer frame and was discharged to specialist neuro-rehabilitation facility for further rehabilitation.
Discussion
Common causes of breathlessness in a postoperative patient generally includes pneumonia, pulmonary embolism, postoperative atelectasis, partial or complete lung collapse, pneumothorax, postoperative pleural effusion, parapneumonic effusion, congestive cardiac failure, severe hypoalbuminaemia, pleural effusion secondary to single-lung ventilation and left-sided thoracotomy, etc.
She was not pyrexial and was not expectorating any productive cough at the time. Her bloods and inflammatory markers were stable, making pneumonia unlikely. In the spinal-orthogeriatric unit, all patients are routinely assessed for venous thrombo-embolism and are on prophylactic enoxaparin, as well as flowtrons to reduce the risk of venous thrombo-embolism, making pulmonary embolism less likely. Her chest radiograph suggested significant pleural effusion, which is not very common in pulmonary embolism. Similarly, in the absence of any known cardiovascular or valvular heart disease and with completely normal electrocardiogram, the possibility of congestive cardiac failure was considered low. Clinical examination was more consistent with pleural effusion rather than pneumothorax or lung collapse, which was subsequently confirmed on diagnostic tap. As she already had a chest drain and was postoperative, the possibility was either para-pneumonic or possible postoperative effusion. Postoperative effusion is mostly seen in the immediate postoperative period and is generally smaller. Her pleural fluid did not show any growth and all her bloods, including inflammatory markers, were stable. Her serum albumin was not significantly low, thus ruling out hypoalbuminaemia as the cause of her pleural effusion. Pleural effusion due to single-lung ventilation and left-sided post-thoracotomy is less likely to develop at four weeks following spinal surgery. However, given signs of intracranial hypotension and associated left-sided effusion following a recent spinal surgery on a giant thoracic disc, the possibility of SPF was strongly suspected, which was subsequently confirmed after her pleural fluid tested positive for β2-transferrin, thus supporting SPF as the cause of her pleural effusion.
SPF is a rare CSF fistula with an overwhelming traumatic nature (blunt/penetrating trauma or post-surgical) but could be of spontaneous aetiology.1,2 It is a rare complication of spinal surgery and is often seen following excision of a giant intradural calcified disc herniation in the thoracic spine, as happened in this case.3
Pleural pressure is generally below atmospheric pressure (from –2 to –8 cm H2O). Subarachnoid space pressure is positive (10–15 cm H2O), and so, in a case of intraoperative dural breach, the CSF will flow from the positive subarachnoid space to the negative pleural space, which becomes even more negative during inspiration. The pleura is able to absorb CSF, but this ability depends on several factors. If the flow of the CSF overwhelms the ability to absorb the pleural fluid, this will lead to pleural collection.4,5
We believe that in our patient recurrent SPF following operation on a giant thoracic intradural disc caused significant cord compression. It is highly likely that the presence of an indwelling chest drain in the immediate postoperative period prevented significant accumulation of pleural effusion from the fistula initially whilst in HDU; however, it started accumulating once the chest drain was removed.
SPF manifests with respiratory and neurological symptoms. Respiratory symptoms include chest pain, dyspnoea, tachypnoea or cough in the presence of profuse pleural effusion.6 Neurological symptoms are similar to those of a CSF leak and include positional headache, vertigo, nausea and development of diplopia after several days or weeks due to paralysis of the sixth cranial nerve as evidence of intracranial hypotension.6
Central nerve system imaging is crucial to rule out a haematoma or cerebellar haemorrhage. As the patient started improving clinically with conservative management whilst on the ward, further brain imaging was not considered essential following discussion with the spinal team who felt her symptoms were typical of cerebral hypotension.
From a laboratory point of view, pleural fluid should be tested for the presence of β2-transferrin, an important biochemical marker with high sensitivity and specificity (94–100%) for the detection of CSF.7 Recurrence is common as seen in this case.
The incidence of dural breach in transthoracic approaches is found to be approximately 15%.8 Obviously, the risk of postoperative CSF leakage is higher with calcified hernias and even higher with giant calcified discs since they often penetrate the dura and their calcified shell adheres to the dura. Gille et al. reported a 39% rate of dural breach after thoracoscopy, but the exact incidence of the SPF after dural breach is not well established.9
Once developed, SPF cannot spontaneously seal itself. In cases of established fistula, treatment is guided by symptoms. A careful monitoring of the cranial symptoms is extremely important and in case of deterioration, application of non-invasive positive pressure ventilation with lumbar CSF drainage has been used successfully in the past.10 Treatment of the fistula generally requires surgical revision to seal the breach with glue and filling of the dead space with muscle or fat tissue. Postoperatively, non-invasive ventilation with 6 cm H2O positive final expiration pressure is recommended, in combination with lumbar drainage.6 Radiologically-guided percutaneous injection of onyx to seal the breach was described by Knafo et al. but this procedure requires identification of the source of the leak.11 In summary, the best treatment for SPF is prevention of the dural breach. Once this has occurred, the dural breach should be meticulously repaired, a lumbar drain should be inserted and non-invasive ventilation techniques should be considered for the patient.
Conclusion
It is important to consider a wider differential when dealing with breathlessness in a postoperative patient. Headache and photophobia in a postoperative spinal patient could point to dural breach and cerebral hypotension. It is worthwhile checking for β2-transferrin in patients with unexplained pleural effusion following spinal surgery.
