Systematic reviews are critical to systematically evaluate the available knowledge to enable the practice of evidence-based medicine. Systematic reviews of randomized controlled trials (RCTs) form the basis of most practice guidelines.1,2 In clinical areas where few RCTs are available, systematic reviews might also be conducted using observational studies.3 In this editorial we have provided an overview of recent trends related to registration, updating and reporting guidelines for systematic reviews. We also provide a framework for a beginner to plan and conduct a systematic review.
Registration of systematic reviews
The first step in planning any systematic review is the development of the review protocol. This includes devising a framework for conducting the systematic review, defining its objectives in the Participants Intervention Comparator Outcomes (PICO) format, and delineating the plan for data presentation and performing a meta-analysis (if warranted and scientifically appropriate).4 A pre-published or pre-registered review protocol enhances the transparency of a systematic review and might help avoid redundancy. Analyses conducted other than those mentioned in the protocol, whether subgroup analyses or sensitivity analyses, should be clearly labelled as post-hoc (since they are more liable to be affected by reporting bias than pre-planned analyses).4 Systematic review protocols are traditionally registered with Cochrane (for Cochrane reviews) or the International Prospective Register of Systematic Reviews (PROSPERO). Currently, registration with PROSPERO might be delayed due to the COVID-19 pandemic. Systematic review protocols can also be pre-published on preprint servers or using the Open Science Framework (osf.io). While protocols registered at such sites might not have been peer reviewed, they do provide a primary checkpoint that peer reviewers can refer to while evaluating the completed systematic review manuscripts. Journals might also publish systematic review protocols. Sometimes researchers avoid such registration due to the mistaken belief that this might result in their idea being usurped by others. However, registered review protocols generally have a date stamp, thereby providing a mechanism of establishing primacy over the idea for the systematic review.5–7
Updating a systematic review
After a systematic review has been published, it might need to be updated at regular intervals in order to include emerging information. Updating of systematic reviews is an important responsibility of the original authors; however, few formal mechanisms exist to facilitate such updating of reviews except for those conducted under the Cochrane collaboration. Future updating of systematic reviews might even be possible using artificial intelligence (AI). However, the use of AI for scanning literature relevant to the PICO objectives of a review is still in its infancy. At this stage, AI is probably more helpful for identifying literature regarding RCTs as opposed to observational studies, and even then, its performance is suboptimal. Another mechanism for identifying relevant literature is crowd sourcing. The Cochrane collaboration through its platform of Cochrane Crowd now regularly utilizes this approach. Crowd sourcing includes a module to train prospective individuals scanning the literature to identify relevant studies to a particular systematic review. Such individuals complete a screening test after the module to ensure that they have understood the objectives of the review before embarking on screening literature for the review.8–12
Reporting guidelines for systematic reviews
The Preferred Reporting Standards for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines from the Enhancing the Quality and Transparency of Health Research (EQUATOR) network were recently updated (PRISMA 2020). Notable changes when compared with the previous PRISMA guidelines published in 2009 are detailed forthwith. It is now recommended to present all the databases searched as well as a detailed search strategy for each database. The use of crowd sourcing or AI for literature searches is also recognised. Reviewers are recommended to also cite those studies that might have been excluded due to the lack of fulfillment of a few inclusion criteria or due to unavailability of complete data despite fulfilling the inclusion criteria. A specific note has been made regarding the assessment and presentation of reporting bias (apart from the usual study quality or risk of bias assessment that had been mandated before). Furthermore, reviewers are encouraged to report certainty of evidence for individual outcomes across studies using methods such as the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. A specific note regarding registration of the review protocol has been incorporated, with a requirement to delineate deviations from the review protocol. However, registration is not mandatory and unregistered review protocols should be mentioned as such. The conflicts of interest section has been expanded to include sources of support and financial/non-financial conflicts of interest. A statement regarding the availability and accessibility of data has also been mandated.13 Overall, these changes enhance the transparency of reporting systematic reviews.
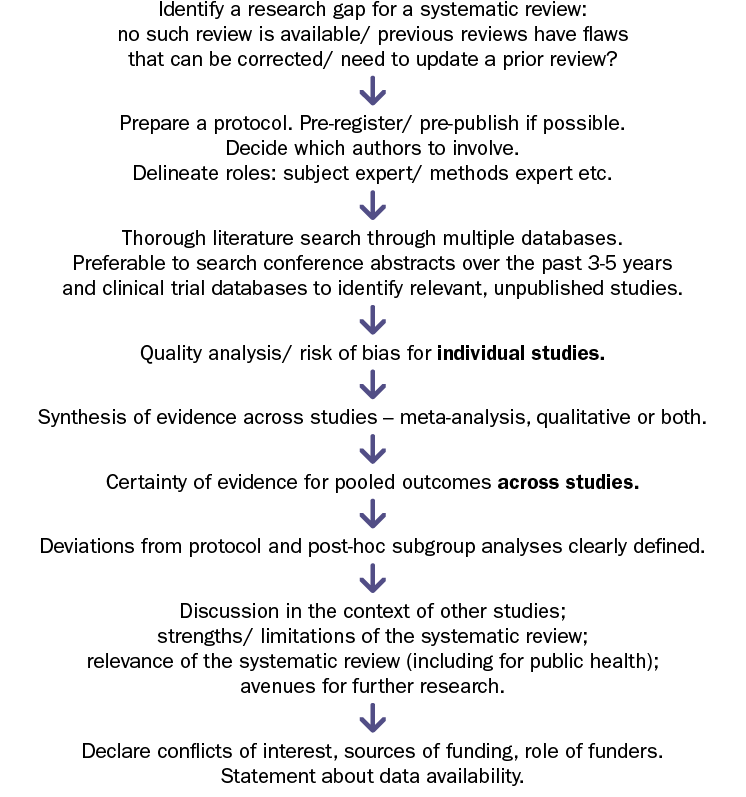
Framework for conducting systematic reviews
In Figure 1, we have presented a framework for conducting systematic reviews. This figure includes the main requirements of the PRISMA 2020 reporting guidelines and is meant to be a primer for the early career researcher just embarking on conducting systematic reviews. It is recommended to further learn the art of conducting systematic reviews through formal courses conducted by the Cochrane collaboration from time to time, reading through the Cochrane Handbook of Systematic Reviews for Interventions,14 as well as by shadowing colleagues who are experienced systematic reviewers.
Figure 1 Framework for conducting systematic reviews
References
1 Misra DP, Agarwal V. Systematic reviews: challenges for their justification, related comprehensive searches, and implications. J Korean Med Sci 2018; 33: 9.
2 Ravindran V, Shankar S. Systematic reviews and meta-analysis demystified. Indian J Rheumatol 2015; 10:89–94.
3 Misra DP, Rathore U, Patro P et al. Disease-modifying anti-rheumatic drugs for the management of Takayasu arteritis—a systematic review and meta-analysis. Clin Rheumatol 2021; Epub ahead of print.
4 Misra DP, Zimba O, Gasparyan AY. Statistical data presentation: a primer for rheumatology researchers. Rheumatol Int 2021; 41: 43–55.
5 Straus S, Moher D. Registering systematic reviews. CMAJ 2010; 182: 13–4.
6 Chang SM, Slutsky J. Debunking myths of protocol registration. Systemat Rev 2012; 1: 4.
7 Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Systemat Rev 2012; 1: 7.
8 Kharawala S, Mahajan A, Gandhi P. Artificial intelligence in systematic literature reviews: a case for cautious optimism. J Clin Epidemiol 2021; Epub ahead of print.
9 Garritty C, Tsertsvadze A, Tricco AC et al. Updating systematic reviews: an international survey. PloS One 2010; 5: e9914.
10 Garner P, Hopewell S, Chandler J et al. When and how to update systematic reviews: consensus and checklist. BMJ 2016; 354: i3507.
11 Koroleva A, Olarte Parra C, Paroubek P. On improving the implementation of automatic updating of systematic reviews. JAMIA Open 2019; 2: 400-401.
12 Martin P, Surian D, Bashir R et al. Trial2rev: combining machine learning and crowd-sourcing to create a shared space for updating systematic reviews. JAMIA Open 2019; 2: 15–22.
13 Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71.
14 Higgins JPT, Thomas J, Chandler J et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester: John Wiley & Sons; 2019.