Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) or COVID-19, constitutes a public health emergency of international concern. The virus has spread globally through aerosol and contact transmission since the discovery of the SARS-CoV-2 in December 2019 in Wuhan, China.1 As of 8 October 2020, 36,002,827 cases of COVID-19 have been reported worldwide, including 1,049,810 deaths.2
The majority of early reported cases had the common symptoms of fever, dry cough and dyspnoea, as well as less common symptoms of headache, myalgia and sputum production. Computerised tomography (CT) scans showed bilateral lung opacities in almost all patients.3 A meta-analysis of risk factors of critical COVID-19 patients showed that patients with dyspnoea were more likely to deteriorate into a critical condition than those who presented with fever only. There was an increased risk among the elderly (especially males over 65 years) and patients with comorbidities, such as diabetes, hypertension, cardiovascular and respiratory disease.4 The assessment of dyspnoea is therefore an essential part of managing patients presenting with suspected COVID-19.
The pandemic has placed increasing strain on scarce healthcare resources such as hospital beds and clinician time. This has been due to both increased demand and the need for stringent infection control procedures. As a result, many countries have relied on primary care systems to reduce the flow of patients through hospital emergency departments.
A large amount of community-based diagnosis and triage of COVID-19 is currently being performed by video and telephone consultation. This has presented clinicians with a new challenge in risk-stratifying patients with shortness of breath. Dyspnoea is a diverse symptom and can be present in those who are critically ill but also in the worried well. Objective modes of assessment are required to differentiate these patient groups.
The transformation of primary care from face-to-face to remote consultations has been aided by technology such as AccuRx, an SMS and video consulting platform in the UK5 and telehealth software such as ExtendedCare in the USA which allowed patients to communicate with clinicians via phone and virtual computer care rooms.6
Guidelines for remote COVID-19 management in primary care have been widely disseminated online between healthcare professionals,7 however, the lack of an in-person assessment and physical examination makes it more difficult to determine which patients need further assessment in secondary care and which can be managed at home. In their paper on remote assessment of COVID-19 in primary care, Greenhalgh et al. recommend asking patients to describe their problem with breathing, to focus on deterioration or change, and to interpret breathlessness in the context of the wider history and physical signs.8 Despite this, there is uncertainty over the optimum risk stratification tools and systems for health professionals to use when remotely assessing these patients.9
To the best of our knowledge, there is currently no widely used and validated tool for remotely assessing dyspnoea in COVID-19.8 Since dyspnoea is also a common presenting symptom in many other conditions, tools designed prior to the COVID-19 pandemic may also be of use. As a result, this review aimed to systematically search the existing literature to identify modalities that can be used to risk-stratify patients presenting with dyspnoea in acute respiratory disorders during remote consultation. We aimed to identify tools that would be of use in the context of acute dyspnoea during COVID-19. As a result, studies limited solely to presentations of chronic respiratory disorders such as chronic obstructive pulmonary disease (COPD) were not considered. Patients with chronic respiratory disorders are triaged and managed differently due to the nature of their condition. For example, patients with COPD or asthma will be assessed using peak expiratory flow rate measurements. They may also have pre-existing plans in place for acute exacerbations that enable them to adjust their medications at home in the initial stages to avoid hospital attendance. These chronic-disease-specific measures have been extensively reviewed prior to the COVID-19 pandemic and were therefore not the focus of this review.10,11
Methods
Protocol and registration
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study protocol was prospectively registered on PROSPERO (ID = CRD42020202292).
Search strategy and selection criteria
A systematic search was performed on 10 June 2020. Medline and Embase via Ovid, medRvix and the Cochrane Database of Systematic Reviews were searched. Searches were limited to publications after January 2000 in order to ensure that telemedicine modalities identified were applicable to current practice. Studies were also limited to English language.
Search terms focussed around three main domains of respiratory distress, triage and telemedicine. Keywords and MeSH terms were combined with standard Boolean operators. The full search strategy is detailed in Appendix 1.
Criteria for inclusion were:
- Studies of the remote assessment of dyspnoea in acute respiratory disorders in adults and children.
- Studies reporting quantitative primary data on any clinical outcome.
- Criteria for exclusion were:
- Studies presenting qualitative data alone.
- Reviews, editorials, letters, commentaries, conference abstracts or methodological articles.
- Studies that report data solely on non-clinical outcomes such as uptake, usability, feasibility or cost.
- Studies of the long-term monitoring of chronic stable respiratory conditions.
- Studies of acute exacerbations of chronic single disease populations.
- Studies primarily of triage modalities requiring in-person assessment.
Titles and abstracts were independently screened by two reviewers, using the ‘Rayyan QCRI’ software.12 The full text of each study passing this stage of screening was then independently screened by the same two reviewers. Disagreements were resolved during a consensus meeting with a third reviewer. At the full-text screening stage, the citation lists of all included studies were searched for papers that were not identified through the database search process.
Data collection and quality assessment
Data extraction was conducted by two reviewers independently and checked by a third reviewer. A standardised data extraction tool was used after being piloted on one study.
Data collected included publication type, study design, triage or stratification method assessed, clinical signs assessed, comparator, population, geographical setting, description and demographics, sample size, focused diseases or conditions and outcomes.
Outcome measures included the sensitivity and specificity of triage modalities where available. The predicted risk of admission, mortality or need for further assessment was also considered where available. This was compared with existing modalities such as in-person assessment or pulse oximetry if possible.
Quality appraisal was conducted using the US National Institute of Health NHLBI’s Study Quality Assessment Tools set, which includes tools for interventional studies, observational studies and case series.13 Two reviewers independently assessed quality using the appropriate tool and came to an overall conclusion of good, fair or poor quality using the NHLBI guidance. Any discordant outcomes were resolved through discussion with a third reviewer.
Data analysis
We performed a narrative synthesis of the studies, evaluating triage modalities, clinical signs and symptoms, and clinical outcomes. Sensitivity and specificity of triage tools for predicting the need for further care were reported where possible. Due to variability in study populations and triage modalities it was not possible to perform a quantitative synthesis.
Results
Our search yielded 3,901 relevant studies. After removing 887 duplicates, 3,014 results were screened by title and abstract against the inclusion and exclusion criteria, providing 32 studies for full-text screening. Ultimately five papers were selected for review (Figure 1).
Figure 1 Prisma flow diagram
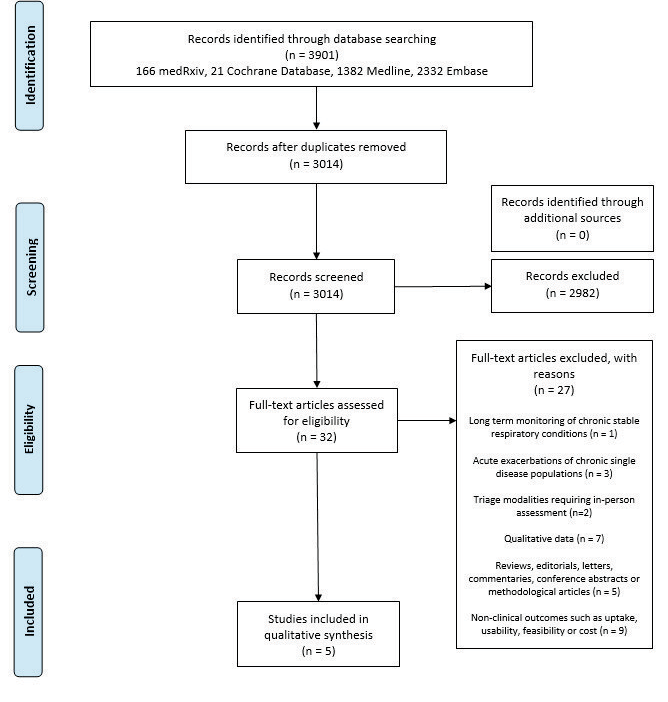
Three cross-sectional studies, one prospective cohort study and one case series were identified. All papers were published between December 2015 and May 2020 and three studies were from the USA. Across all studies, a total of 1,317 patients were triaged using telephone-related modalities, video consultation or internet-based self-triage tool. A summary of the included studies is provided in Table 1.
Table 1 Summary of included studies
|
|
|
|
|
|
|
|
|
|
Clinical Cardiology
|
Pediatrics
|
Journal of the American Medical Informatics Association
|
Journal of Korean Medical Sciences
|
Hospital Pediatrics – American Academy of Pediatrics
|
|
|
01.08.2016
|
10.12.2015
|
13.05.2020
|
09.04.2020
|
01.08.2016
|
|
|
Israel
|
USA
|
USA
|
South Korea
|
USA
|
|
|
|
Cross-sectional study
|
Cross-sectional study
|
Prospective cohort study
|
Case series
|
Cross-sectional study
|
|
|
93
|
145
|
950
|
3,033
|
48
|
|
|
Mean 76 years
|
2 months to 18 years (4.7 years)
|
(42 years)
|
–
|
8 months to 18 years
|
|
|
Verbal Roth Score*
|
Video-based ROC2
|
Online UCSF coronavirus symptom checker***
|
Telephone remote severity scoring system***
|
Video-based respiratory score**
|
|
|
Pulse oximetry
|
Face-to-face observation
|
–
|
–
|
Face-to-face observation
|
|
|
Hospital inpatients with hypoxia
|
Children presenting with cough, dyspnoea or wheeze
|
Patients with symptoms of COVID-19
|
Patients with confirmed COVID-19
|
Children presenting with respiratory distress
|
|
|
|
Counting time >8 seconds had a sensitivity of 78% and specificity of 73% for predicting pulse oximetry <95%
|
Weighted kappa of 0.85 for agreement on presence of respiratory distress by telemedicine vs bedside evaluation
|
Sensitivity for detecting emergency-level care use was 87.5% (95% CI 61.7–98.5%)
|
Positive predictive value of 97.3% for determining which patients did not require hospital admission
|
Sensitivity 83% and specificity 84% for correctly diagnosing ‘severe’ respiratory distress using telemedicine
|
|
|
Roth score is a quick and easy to use surrogate measure for hypoxia that can be combined with telemedicine
|
The ROC used via telemedicine is a reliable tool to detect respiratory distress in children
|
Online patient self-triage tools can prevent unnecessary visits during the COVID-19 pandemic and increase triage efficiency
|
Remote telephone triage of COVID-19 severity can help reduce shortages of hospital beds
|
Telemedicine may be an effective and reliable tool for the remote assessment of respiratory distress in children
|
A summary of the clinical triage parameters identified within the studies is provided in Table 2. Parameters included respiratory signs/symptoms, non-respiratory signs/symptoms, and patient characteristics. The most commonly included parameters were respiratory rate and altered mental status.
Table 2 Clinical characteristics observed in included studies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Counting time*
|
|
X
|
|
X
|
|
|
Respiratory rate**
|
X
|
X
|
X
|
|
|
|
Work of breathing***
|
X
|
X
|
|
|
|
|
Cough
|
|
|
X
|
|
X
|
|
Wheeze
|
|
X
|
|
|
|
|
|
|
Altered mental status
|
X
|
X
|
|
|
X
|
|
Reduced oral intake
|
|
X
|
|
|
X
|
|
Fever
|
|
|
X
|
|
X
|
|
Perioral cyanosis
|
X
|
|
|
|
X
|
|
Lethargy
|
|
|
|
|
X
|
|
Chest pain
|
|
|
|
|
X
|
|
|
|
Comorbidities†
|
|
|
X
|
|
|
|
Immunocompromise
|
|
|
X
|
|
X
|
|
Age
|
|
|
X
|
|
|
|
Social factors††
|
|
|
X
|
|
|
Two studies used a video-based remote symptom scoring method to identify children with respiratory distress, with both measuring the agreement of telemedicine evaluation with simultaneous face-to-face observations. Gattu et al. found telemedicine to be an effective tool with a sensitivity of 83% and specificity of 84% for diagnosing ‘severe’ respiratory distress.18 Severe was defined as a score greater than or equal to 9 on the 12-point respiratory score.18 Siew et al. found ‘excellent’ agreement between telemedicine and bedside observation when using the de novo Respiratory Observation Checklist (ROC), defined by a weighted kappa of 0.85 for determining the presence of respiratory distress.15
A further two studies investigated a remote method to triage suspected cases of COVID-19. Kim et al. used a telephone scoring system to classify patients as mild, moderate, severe or critical COVID-19 severity.17 Mild or moderate severity patients without comorbidities were not admitted to hospital, with only 2.67% of such cases requiring subsequent hospital admission.17 Judson et al. described an online self-triage tool for COVID-19 patients assessing dyspnoea, concurrent symptoms and comorbidities, which demonstrated a sensitivity of 87.5% for identifying COVID-19 patients who subsequently required emergency level care.16
Finally, Chorin et al. used the Roth Score as a potential telephone-based risk-stratification method for detecting patients with hypoxia. The study showed that for patients who took longer than eight seconds to count to 30, the test had a sensitivity of 78% and a specificity of 73% for detecting pulse oximetry values of <95%.14
In the quality assessment, we evaluated the overall quality of two studies as good, two as fair and one as poor (Table 3). Studies generally performed poorly at reporting participation rates. Selection bias was a problem across multiple studies as random or stratified sampling was not employed. Confounding factors such as patient demographics and disease severity were also not controlled for in most studies. However, studies did well at defining research questions and blinding assessors.
Table 3 Summary of quality assessment of included studies
|
|
|
|
|
|
|
|
|
|
|
|
1. Was the research question or objective in this paper clearly stated?
|
Y
|
Y
|
Y
|
Y
|
1. Was the study question or objective clearly stated?
|
Y
|
|
2. Was the study population clearly specified and defined?
|
Y
|
Y
|
N
|
Y
|
2. Was the study population clearly and fully described, including a case definition?
|
N
|
|
3. Was the participation rate of eligible persons at least 50%?
|
CD
|
CD
|
N
|
CD
|
3. Were the cases consecutive?
|
Y
|
|
4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants?
|
Y
|
Y
|
Y
|
Y
|
4. Were the subjects comparable?
|
CD
|
|
5. Was a sample size justification, power description, or variance and effect estimates provided?
|
N
|
N
|
N
|
Y
|
5. Was the intervention clearly described?
|
Y
|
|
6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured?
|
N
|
N
|
Y
|
N
|
6. Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants?
|
N
|
|
7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed?
|
N
|
N
|
Y
|
N
|
7. Was the length of follow up adequate?
|
N
|
|
8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)?
|
Y
|
Y
|
Y
|
Y
|
8. Were the statistical methods well-described?
|
N
|
|
9. Were the exposure measures (independent variables) clearly defined, valid, reliable and implemented consistently across all study participants?
|
Y
|
Y
|
Y
|
Y
|
9. Were the results well described?
|
N
|
|
10. Was the exposure(s) assessed more than once over time?
|
N
|
N
|
N
|
N
|
|
|
|
11. Were the outcome measures (dependent variables) clearly defined, valid, reliable and implemented consistently across all study participants?
|
Y
|
Y
|
Y
|
Y
|
|
|
|
12. Were the outcome assessors blinded to the exposure status of participants?
|
Y
|
Y
|
Y
|
Y
|
|
|
|
13. Was loss to follow up after baseline 20% or less?
|
NA
|
NA
|
Y
|
NA
|
|
|
|
14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?
|
N
|
N
|
N
|
N
|
|
|
|
Overall impression
|
Fair
|
Fair
|
Good
|
Good
|
Overall impression
|
Poor
|
|
Final score
|
7
|
7
|
9
|
8
|
Final score
|
3
|
Discussion
Remote risk stratification enables a more effective allocation of resources and works to prevent unnecessary travel and mixing of individuals when the risk of contagion is high. This is especially useful amongst rural or comorbid patient groups who are less able to travel. The COVID-19 pandemic necessitated a rapid transformation of the treatment of patients, with a significant proportion of triage and risk-stratification being performed via telephone and video consultations. This occurred in the absence of robust evidence for the optimal methods of assessing dyspnoea remotely. Our study aimed to address this gap by identifying and summarising existing tools for the remote assessment of dyspnoea. This is essential to ensure that future triage of patients presenting with dyspnoea is accurate, efficient and safe. Our findings will inform the development and validation of focussed tools for the assessment of acute dyspnoea within the context of COVID-19.
Existing tools for assessing dyspnoea in suspected COVID-19 include the published guidance by Greenhalgh et al. for virtual consultations.8 The paper proposes a series of questions to help distinguish the severely ill from the mildly ill, across themes such as:
- Change in symptoms, e.g. “What makes you breathless now that didn’t make you breathless yesterday?”.
- Limitation of function, e.g. “Are you so breathless that you are unable to speak more than a few words?” .
- Associated symptoms and signs (or ‘red flags’), e.g. chest pain, audible wheeze, cyanosis, feeling cold, clammy or confused.
- Comorbidities, e.g. asthma or cardiovascular disease.
It is important to note that this guidance was formed using the expert opinions of 50 UK-based professionals and has not been validated empirically. Similar unvalidated tools also exist throughout the grey literature. Our review identifies data to inform the use of many of these remote triage elements.
For example, Judson et al. describe the use of an online self-triage tool that categorised suspected COVID-19 patients as ‘emergent,’ requiring immediate care, if they reported any of ‘chest pain, severe shortness of breath, bluish lips or face or confusion’. A total of 950 patients accessed this tool, with 16 patients requiring emergency department level care within 48 hours.16 Of these, 14 patients (87.5%) had already been triaged as ‘emergent’ due to the presence of the aforementioned red flags. However, it should be noted that 193 patients in total were triaged to the ‘emergent’ category, suggesting that the use of these red flag symptoms may overestimate the need for emergency care. Additionally, the lack of a control group and limited population demographics make it difficult to interpret the relevance of the online tool outside of the study population.
In contrast, Kim et al. implemented a telephone severity scoring system for COVID-19 patients that recommended hospital admission for patients with comorbidities such as active cancer, end-stage renal failure, COPD, congestive heart failure or iatrogenic immunosuppression.17 Only 2.67% of 3,033 patients without these comorbidities subsequently required hospital admission. However, it was not possible to calculate the sensitivity of this tool as the number of patients correctly identified as requiring hospital admission was not provided. Therefore, it is difficult to determine the net effect of this remote tool on resource utilisation.
Owing to the paucity of data specific to COVID-19, it is necessary to look to other acute respiratory disorders. Tools such as the Roth Score can be easily applied using telemedicine to patients with suspected hypoxia. However, the Roth Score was validated in hospital inpatients, using face-to-face assessment alone.14 The efficacy of using the Roth score in primary care and via telephone remains to be seen.
The international response to COVID-19 is dynamic, with each nation differing in their access to technologies and population demographics. Our study is useful in identifying research across multiple technological mediums, including telephone, internet and video-based triage modalities. This ensures that a diverse mix of approaches can be considered when developing future triage tools. The strengths and weaknesses of each of these approaches are considered in Table 4.
Table 4 Comparison table of remote triage modalities
|
|
|
|
|
Internet based self-triage tool
|
- Trained professional not required
- Able to access 24/7 and complete as many times as patient desires
- Simple and quick to complete, so greater uptake
|
- Patient may not input sufficient or correct data
- Automated nature requires lower threshold for escalation, which may result in unnecessary use of clinical resources
|
|
Telephone consultation
|
- Minimal technical difficulties
- Patient is able to speak directly to healthcare professional and voice concerns directly
- Triage completed by experienced healthcare professional
- Real-time communication and ability to build rapport
|
- Unable to pick up on non-verbal cues
- Unable to make visual observations of respiratory distress and general health
|
|
Video Consultation
|
- Healthcare professional has visual and audio input from patient for better informed triage
- Triage completed by experienced healthcare professional
- Real-time communication and ability to build rapport
|
- Correct equipment and high-quality internet connection required
- May be more difficult to access for certain population groups
- Can be expensive and cumbersome for healthcare provider
|
The three remote triaging modalities have been employed in the context of the COVID-19 pandemic. A user-friendly self-triage screening tool, such as that designed by Schrager et al., can reduce the workload for healthcare professionals, who need not be a primary part of the triage process.19 Assessment through telephone consultations are rated highly for their ease of use and increased accessibility, as shown by the tool developed and utilised by Elkin et al.20 Video consultations appear to be particularly useful in removing barriers to access for certain groups seeking medical assessment and advice. Greenhalgh et al. note the greater reassurance of a video consultation when compared to telephone, as well as the benefits for people who are frail or immunocompromised.21
Existing systematic reviews of risk stratification in COVID-19 include a study of 145 prediction models by Wynants et al.22 They describe diagnostic models for detecting COVID-19 and prognostic models for predicting outcomes. As with our review, they found a high risk of bias amongst included studies due to factors such as incomplete reporting or inadequate control groups. Ultimately, they did not recommend the clinical use of any particular tool, but did identify candidate predictors for future models. Our study differs by focussing on the assessment of dyspnoea rather than the diagnosis of COVID-19. Additionally, our study is concentrated on remote triage modalities which do not rely on radiological or laboratory investigations. Therefore, the studies from our review are of particular relevance to primary care clinicians who need to make quick and accurate decisions about emergency department attendance for patients presenting with varying degrees of dyspnoea.
Monaghesh et al. identified eight studies in their review of telehealth approaches to routine patient care during COVID-19.23 Of note, they found that telehealth tools were often more useful when integrated with electronic health records or social media. Interestingly, this approach was not adopted by any of the studies in our review, likely due to concerns over privacy and data security. Also, integration with existing software may be of more value for chronic conditions than for the assessment of acute dyspnoea.
Many of the studies within this review describe triage modalities for specific patient groups, such as patients with suspected COVID-19 or hospital inpatients with hypoxia. Some parameters are common to different disease processes, such as those outlined in Table 2. However, other elements are difficult to extrapolate to the assessment of dyspnoea as a whole, so caution must be applied when interpreting the results. For example, anosmia and ageusia were recently detected as pertinent presenting symptoms of COVID-19 but are less relevant to other causes of acute dyspnoea.24 Ultimately future tools developed for the assessment of dyspnoea will require tailored evaluation for the population they are intended for.
There are several limitations to this review. Firstly, English language studies from only three countries were examined. This limits the applicability of our conclusions to patient populations in different healthcare settings. Secondly, the studies varied in their methodology, patient groups and outcomes. Many studies were of only poor or fair quality which limited statistical comparison across studies. Finally, due to the rapidly evolving nature of the COVID-19 pandemic, new research is emerging daily and will not be included in this review. However, this study is useful in assessing literature from before the pandemic.
Further work is needed to develop an accurate and validated remote triage tool for dyspnoea in acute respiratory disorders such as COVID-19. Suggestions are outlined below for future studies:
- Consider the strengths and weaknesses of different triage modalities (Table 4) and ensure the method chosen is appropriate for the population of interest.
- Consider the inclusion of clinical parameters such as those outlined in Table 2.
- Ensure that the proposed triage modality is compared to a suitable control, preferably face to face assessment.
- Aim to record correlation with clinical endpoints, or objective markers such as pulse oximetry where this is not possible.
- Ensure that results are reported in a consistent and easily interpreted manner, such as sensitivity, specificity and area under the ROC curve.
The emergence of an effective, evidence-based tool to assess dyspnoea via telemedicine will prove vital in reducing the demand on health systems and containing the spread of disease.
This study identifies a range of remote risk stratification tools for assessing acute dyspnoea severity across video, telephone and online mediums. Clinical features from these tools can be explored for use during COVID-19 to prevent unnecessary hospital attendances. Although no optimal remote risk stratification tool has been identified, this review will inform the development of future risk stratification tools. 
Online Supplementary Material
Supplementary data are available with the online version of this paper, which can be accessed at https://www.rcpe.ac.uk/journal
References
1 Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol 2020; 92: 548–51.
2 COVID WHO. Dashboard [Internet]. https://covid19.who.int/ (accessed 23/04/20).
3 Jiang F, Deng L, Zhang L et al. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med 2020; 1–5.
4 Zheng Z, Peng F, Xu B et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 2020; 81: e16–e25.
5 Khan N, Jones D, Grice A et al. A brave new world: the new normal for general practice after the COVID-19 pandemic. BJGP Open 2020; 4: bjgpopen20X101103.
6 Wosik J, Fudim M, Cameron B et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Informatics Assoc 2020; 27: 957–62.
7 Joy M, McGagh D, Jones N et al. Reorganisation of primary care for older adults during COVID-19: a cross-sectional database study in the UK. Br J Gen Pract 2020; 70: e540–7.
8 Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ 2020; 368: m1182.
9 Knight SR, Ho A, Pius R et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ 2020; 370.
10 Baroi S, McNamara RJ, McKenzie DK et al. Advances in remote respiratory assessments for people with chronic obstructive pulmonary disease: a systematic review. Telemedicine e-Health 2018; 24: 415–24.
11 Kew KM, Cates CJ. Remote versus face-to-face check-ups for asthma. Cochrane Database of Systematic Reviews 2016; 4: CD011715.
12 Rayyan QCRI. 2020. https://rayyan.qcri.org/users/edit (accessed 21/9/21).
13 National Heart, Lung, and Blood institute. Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed 9/9/21).
14 Chorin E, Padegimas A, Havakuk O et al. Assessment of respiratory distress by the Roth score. Clin Cardiol 2016; 39: 636–9.
15 Siew L, Hsiao A, McCarthy P et al. Reliability of telemedicine in the assessment of seriously ill children. Pediatrics 2016; 137: e20150712.
16 Judson TJ, Odisho AY, Neinstein AB et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Informatics Assoc 2020; 27: 860–6.
17 Kim S-W, Lee KS, Kim K et al. A brief telephone severity scoring system and therapeutic living centers solved acute hospital-bed shortage during the COVID-19 outbreak in Daegu, Korea. J Korean Med Sci 2020; 35: e35.
18 Gattu R, Scollan J, DeSouza A et al. Telemedicine: a reliable tool to assess the severity of respiratory distress in children. Hosp Pediatr 2016; 6: 476–82.
19 Schrager JD, Schuler K, Isakov AP et al. Development and usability testing of a web-based COVID-19 self-triage platform. West J Emerg Med 2020; 21: 1054.
20 Elkin E, Viele C, Schumacher K et al. A COVID-19 screening tool for oncology telephone triage. Support Care Cancer 2020; 1–6.
21 Greenhalgh T, Wherton J, Shaw S et al. Video consultations for covid-19. BMJ 2020; 368: m998.
22 Wynants L, Van Calster B, Bonten MMJ et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ 2020; 369.
23 Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health 2020; 1193.
24 Vaira L, Salzano G, Deiana G et al. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 2020; 130: 1787.
