Amiodarone lung presenting as acute fulminant fatal pneumonitis despite long-term use of a very low dose regimen is rarely reported. Treating clinicians should have a high degree of suspicion as amiodarone lung can sometimes be a diagnostic challenge. Appropriate clinical vigilance irrespective of amiodarone dose and duration remains the cornerstone in preventing this condition.
Case presentation
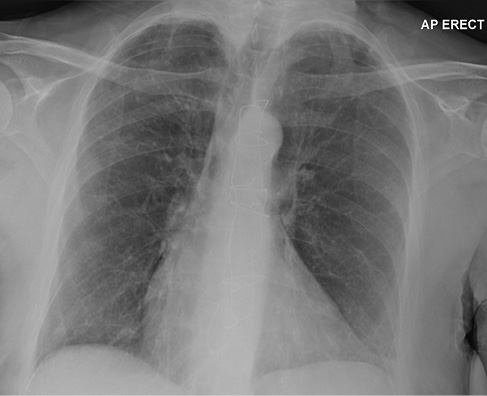
A 68-year-old female presented with a 2-week history of worsening exertional breathlessness, unproductive cough and orthopnoea. The patient had a past history of cerebrovascular disease, patent foramen ovale repair, tricuspid valve annuloplasty and difficult to control atrial fibrillation requiring amiodarone 100 mg once daily since March 2010. On examination the patient was febrile (38.5 °C) with evidence of raised jugular venous pressure, a soft systolic murmur in the tricuspid area and bilateral crepitations with no ankle swelling. Blood tests showed leucocytosis, transaminitis, elevated C-reactive protein and the chest X-ray (CXR) was abnormal (Figure 1). The patient’s previous CXR from 2016 was normal (Figure 2). Arterial blood gas carried out on 10 L oxygen showed hypoxic respiratory failure (PH: 7.42, PCO2: 4.5 kpa, PO2: 6.7 kpa). The patient was initiated on nasal high flow (NHF), broad spectrum antibiotics and diuretics.
Figure 1 Chest X-ray from September 2019. Widespread bilateral airspace opacification.
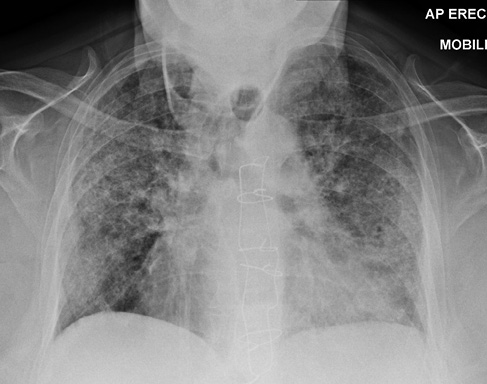
Figure 2 Chest X-ray from June 2016. Relatively clear lung fields with sternotomy and evidence of tricuspid valve surgery.
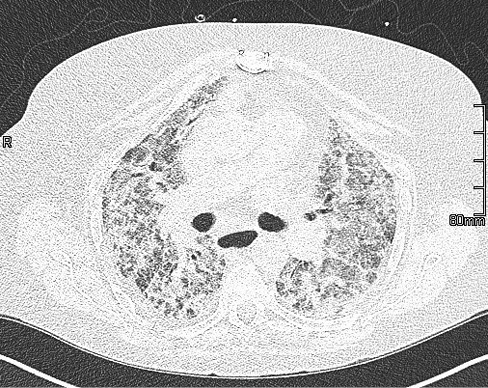
Legionella (group 1), pneumococcal antigen, blood and urine cultures, procalcitonin, autoimmune screen, immunoglobulins, HIV, avian IgG precipitants and atypical serology were negative. Echocardiogram showed good left ventricular function, mild tricuspid regurgitation and raised pulmonary artery pressure (45 mmHg). Abdominal ultrasound showed focal area of fatty infiltration of the liver. The patient had a suboptimal response to medical therapy and had worsening hypoxia. AIPT was considered and thus the patient had a high-resolution CT chest (Figure 3). The differential diagnosis at this stage was either idiopathic acute interstitial pneumonitis or amiodarone-induced pneumonitis. The patient had 3 days of pulsed methylprednisolone and NHF changed to continuous positive airway pressure (positive end expiratory pressure: 10 cm of water, FiO2: 90%) with no significant improvement in clinical parameters. The patient was high risk for invasive interventions and was reviewed by the intensive care team who deemed the patient not for escalation to level 3 care. The patient opted for a palliative care approach and active treatment was withdrawn.
Figure 3 High-resolution CT from September 2019. Bilateral extensive parenchymal abnormalities comprising predominantly ground-glass opacification with some interlobular septal thickening but no traction bronchiectasis/fibrosis; suggestive of an acute pneumonitis.
The gross findings at postmortem showed a normal upper respiratory tract. The lungs were heavy (right lung: 1031 g, left lung 881 g) with diffuse solid appearance with a red–brown cut surface. There were no other focal lesions seen. Microscopic findings (Figure 4) revealed a consolidated and congested lung with loss of normal tissue architecture, hyperplasia of type II pneumocyte with extensive areas showing vacuolation of the cytoplasm of the alveolar pneumocytes and bronchial epithelium. There were abundant foamy macrophages (Figure 5) with focal areas showing hyaline membrane formation suggestive of diffuse lung damage. Attempted electron microscopic studies yielded limited outcome owing to poor tissue preservation. Despite intensive examination cytoplasmic lamellated bodies could not be identified. Given the clinical context the findings were highly suggestive of AIPT.
Figure 4 Consolidated and congested lung tissue with loss of normal alveolar spaces.
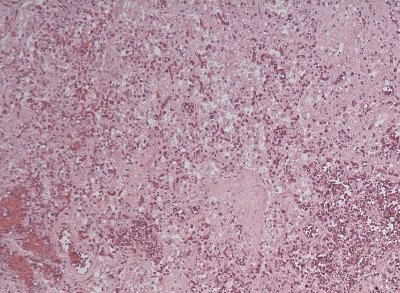
Figure 5 Foamy macrophages (blue arrow) filling alveolar spaces.
Discussion
Amiodarone is used both as an effective antiarrhythmic agent and also prophylactically in the perioperative period in patients undergoing various invasive interventions.1,2 Depending on the dose and duration, both pulmonary and extrapulmonary side effects (thyroid, gastrointestinal, hepatic, cardiac, ophthalmic and dermatological) have been reported.3
AIPT, the most serious of all side effects, has a reported incidence of up to 17% and can be caused by direct cytotoxic effect or by immunological reaction.3,4 Males, increasing age, chronic lung disease, exposure to high concentration of oxygen or during invasive ventilation, and a high total cumulative dose rather than the daily dose or plasma concentration of the medication increases the risk for AIPT.5–7 Although there is no correlation with the duration of treatment, patients with greatest risk are those who are treated with 400 mg or more for more than 2 months or 200 mg for more than 2 years on a daily basis.6 However, it is important to note that there is no safety net on the dose and toxicity can occur at any point during treatment or even after stopping,8 thus the need for appropriate clinical vigilance. A European survey and a recent meta-analysis concluded that the use of ‘very-low-dose amiodarone’, defined as 100 mg/day or less, is associated with lower incidence of side effects than with a higher dose.9,10
Patients may present with subacute onset of progressive dyspnoea, which is often the dominant symptom, malaise, fever, non-productive cough and occasionally pleuritic chest pain. AIPT may also present an indolent course particularly in fibrotic disease.6 Haemoptysis is usually due to diffuse alveolar haemorrhage, which is a rare entity in AIPT. Respiratory failure at presentation is very rare, often mimicking other clinical syndromes similar to our case and usually suggests acute pneumonitis. Clinical examination may be unremarkable in early disease and in progressive stages; hypoxia, respiratory distress and fine crepitations may be noted.
Leucocytosis, eosinophilia, lactic dehydrogenase and a mucin-like glycoprotein known as KL-6 may be elevated.6,11 Chest radiographic features are non-specific and may show bilateral infiltrates especially in the right upper lobe often mimicking pulmonary infection in a third of patients or features suggestive of underlying interstitial disease.12 High-resolution CT is the modality of choice and often reveals bilateral changes associated with either ground-glass or interstitial changes.12 Peripheral ground-glass changes may be an early finding in AIPT and established fibrosis may develop in up to 7% of patients following acute pneumonitis.6 Diffuse attenuation due to high iodine content may also be seen in liver and spleen. This feature is also seen in patients exposed to amiodarone as well.12 Rarely AIPT presents as solitary pulmonary nodules, often in the upper lobes with increased fluorodeoxyglucose uptake mimicking lung cancer.13 Pulmonary function often reveals a restrictive ventilatory defect with reduced lung volumes and reduced carbon monoxide diffusion capacity (DLCO). A 15% drop in DLCO is felt to be significant when monitoring patients for pulmonary toxicity.14 Bronchoalveolar lavage (BAL) may be useful in excluding infective causes and malignancy. Polymorphs and CD8 T cells are often seen in excess in the BAL fluid. Foamy macrophages may be seen in BAL fluid and this is not diagnostic of AIPT as these can be seen in half of the patients on amiodarone with no evidence of toxicity.
Histological features on lung biopsy show diffuse interstitial pneumonitis, less frequently interstitial fibrosis, bronchiolitis obliterans organising pneumonia and diffuse alveolar damage with hyaline membrane formation.15 Although not specific to AIPT, the presence of lipid-laden foamy macrophages and membrane-bound lamellar bodies similar to surfactant on electron microscopic studies are characteristic findings.15
If AIPT is suspected, early cessation of amiodarone should be considered. The resolution of symptoms and radiological changes may be slow and variable owing to the long half-life of the drug. Changes seen in chest radiographs can take up to 18 months to completely resolve.14 Current evidence for the use of steroids is not strong but may be considered on a case-by-case basis with a risk : benefit assessment and discussion with the patients. Systemic corticosteroids may be considered, a dose of 40–60 mg daily for a period of up to 12 months to avoid relapse.16 In patients with acute pneumonitis intravenous methylprednisolone followed by oral prednisolone may be considered to improve gas exchange. Relapse following early steroid withdrawal especially in patients with increased body mass index has been reported owing to high concentration of lipophilic amiodarone within their adipocytes.17 Role of vitamin E in attenuating AIPT has been postulated in animal models.18
The prognosis is favourable in milder cases of AIPT but the prognosis is poor in patients who present with acute fulminant pneumonitis. A proper clinical risk assessment and appropriate patient education along with clinical vigilance is paramount in preventing AIPT or recognising it early. Current guidelines19 suggest all patients have a baseline CXR and a pulmonary function test prior to commencing amiodarone. Patients should be regularly monitored for respiratory symptoms along with other system side effects. Annual CXR along with thyroid and liver function tests should be considered during treatment phase. For patients with suspected AIPT, immediate cessation of the drug along with further assessment and referral to specialist services should be considered.
Conclusion
In conclusion, despite the practice of a ‘very-low-dose amiodarone’ regimen one should anticipate the associated multifaceted complications. Regular medication vigilance and monitoring is vital for a favourable patient outcome. 
References
1 Primeau R, Agha A, Giorgi C et al. Long term efficacy and toxicity of amiodarone in the treatment of refractory cardiac arrhythmias. Can J Cardiol 1989; 5: 98–104.
2 Aasbo JD, Lawrence AT, Krishnan K et al. Amiodarone prophylaxis reduces major cardiovascular morbidity and length of stay after cardiac surgery: a meta-analysis. Ann Intern Med 2005; 143: 327–36.
3 Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation 2012; 125: 381–9.
4 Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J 2009; 16: 43–8.
5 Rotmensch HH, Belhassen B, Swanson BN et al. Steady-state serum amiodarone concentrations: relationship with antiarrhythmic efficacy and toxicity. Ann Intern Med 1984; 101: 462–9.
6 Camus P, Martin WJ II, Rosenow EC III. Amiodarone pulmonary toxicity. Clin Chest Med 2004; 25:65–75.
7 Ashrafian H, Davey P. Is amiodarone an under recognized cause of acute respiratory failure in the ICU. Chest 2001; 120: 275–82.
8 Polkey MI, Wilson PO, Rees PJ. Amiodarone pneumonitis: no safe dose. Respir Med 1995; 89: 233–5.
9 Blackman DJ, Ferguson JD, Bashir Y. Dose of amiodarone in atrial fibrillation—neither guidelines nor clinical practice reflect available evidence. Eur Heart J 2002; 23: 908.
10 Chokesuwattanaskul R, Shah N, Chokesuwattanaskul S et al. Low-dose amiodarone is safe: a systematic review and meta-analysis. J Innov Cardiol Rhythm Manag 2020; 11: 4054–61.
11 Endoh Y, Hanai R, Uto K et al. Diagnostic usefulness of KL-6 measurements in patients with pulmonary complications after administration of amiodarone. J Cardiol 2000; 35: 121–7.
12 Maffessanti M, Polverosi R, Dalpiaz G et al. Diffuse Lung Diseases. Milan: Springer-Verlag; 2006.
13 Jarand J, Lee A, Leigh R. Amiodaroma: an unusual form of amiodarone-induced pulmonary toxicity. CMAJ 2007; 176: 1411–3.
14 Papiris SA, Triantafillidou C, Kolilekas L et al. Amiodarone. Drug Safety 2010; 33, 539–58.
15.Myers JL, Kennedy JI, Plumb VJ. Amiodarone lung: pathologic findings in clinically toxic patients. Hum Pathol 1987; 18: 349–54.
16 Yamada Y, Shiga T, Matsuda N et al. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J 2007; 71: 1610–6.
17 Okayasu K, Takeda Y, Kojima J et al. Amiodarone pulmonary toxicity: a patient with three recurrences of pulmonary toxicity and consideration of the probable risk for relapse. Intern Med 2006; 45: 1303–7.
18 Card JW, Racz WJ, Brien JF et al. Attenuation of amiodarone induced pulmonary fibrosis by vitamin E is associated with suppression of transforming growth factor-beta1 gene expression but not prevention of mitochondrial dysfunction. J Pharmacol Exp Ther 2003; 304: 277–83.
19 Goldschlager N, Epstein AE, Naccarelli GV et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm 2007; 4: 1250–9.
