Case presentation
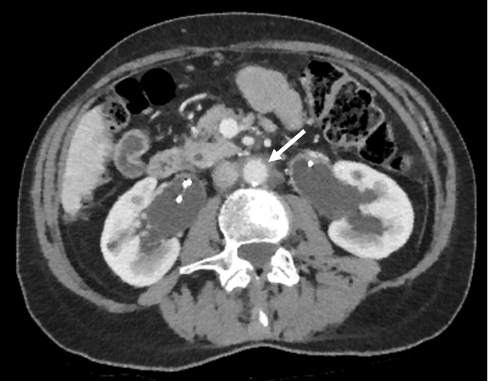
A 65-year-old female was seen in the nephrology clinic in May 2018 for evaluation and management of newly diagnosed retroperitoneal fibrosis (RPF). She initially presented to her general practitioner with a 6-month history of nonspecific constitutional symptoms, malaise and unintentional weight loss. She denied any urinary tract symptoms or pain in her back, flank or abdomen. Her past medical history included atrial fibrillation and well-controlled rheumatoid arthritis. Her regular medications comprised bisoprolol and dabigatran; methotrexate had been stopped several years before. Clinical examination was unremarkable. Routine blood results showed a white cell count of 7.5 × 109/l (reference range: 4–10 × 109/l), haemoglobin 123 g/l (120–150 g/l), platelets 270 × 109/l (150–410 × 109/l), C-reactive protein (CRP) 7 mg/l (0–10 mg/l) and erythrocyte sedimentation rate (ESR) 8 mm/hour (1–12 mm/hour). Her kidney function had deteriorated slightly with a rise in serum creatinine from a baseline of 61 µmol/l (45–84 µmol/l) to 124 µmol/l. CT scan of the thorax, abdomen and pelvis with contrast showed a large retroperitoneal soft tissue mass encasing the aorta and ureters with bilateral hydronephrosis (Figure 1), but no other significant pathology. She underwent urgent bilateral ureteric stent insertion, and her renal function improved significantly with her creatinine falling to 82 µmol/l. Further investigations to seek an underlying cause for her RPF included tumour markers (carcinoembryonic antigen, CA125, CA19-9), immunoglobulin G subclasses, antinuclear antibodies (ANA) and antineutrophil cytoplasmic antibodies (ANCA); all were normal. Biopsy of the retroperitoneal mass was considered but was felt to be too high risk. A diagnosis of idiopathic RPF was thought most likely and oral prednisolone 40 mg daily was commenced with a plan to taper over the next few months, guided by clinical response and imaging. Repeat CT in October 2018 revealed significant regression of the inflammatory mass in keeping with a good response to corticosteroid therapy (Figure 2).
Figure 1 CT scan showing bilateral hydronephrosis (black arrows) and soft tissue mass surrounding the aorta (white arrow)
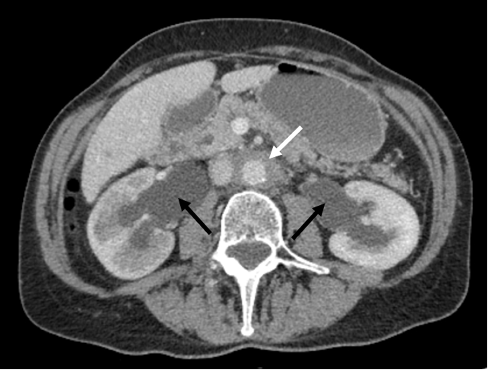
Figure 2 CT scan showing reduction in size of periaortic mass (white arrow)
Discussion
RPF is a rare condition with an incidence of around 1.3 per 100,000 per year in one study.1 It is characterised by a fibroinflammatory process within the retroperitoneal space, most commonly centred around the abdominal aorta (usually infrarenal) and iliac arteries, leading to entrapment of adjacent structures, such as the ureters, inferior vena cava and other organs.2 The disease was first recognised in 1905 by the French urologist Albarran, and was described further in 1948, being known as Ormond’s disease since then.3,4
The majority of cases of RPF (>70%) are idiopathic (primary), with the remainder being secondary to other causes including drugs, infection, malignancy, previous surgery and radiotherapy (Box 1).2 Chronic periaortitis, where fibrosis develops around an inflammatory abdominal aortic aneurysm, can cause to RPF.2,3 More recently, it has also been found to be associated with autoimmune disorders, the commonest being IgG4-related disease, a chronic inflammatory condition characterised by diffuse infiltration of IgG4-secreting plasma cells into various organs.2,5 Other immune-mediated diseases associated with RPF include rheumatoid arthritis, systemic vasculitis and Hashimoto’s thyroiditis.2
Box 1 Causes of retroperitoneal fibrosis
|
Idiopathic (70%), with or without IgG4-related disease
Secondary
- Inflammatory periaortitis around an atherosclerotic aneurysm
- Drugs e.g. methysergide, bromocriptine, methyldopa, hydralazine, beta blockers
- Malignancy e.g. lymphoma, sarcoma, carcinoid tumour, colon, pancreas, prostate, bladder, breast cancer
- Chronic infection: tuberculosis, histoplasmosis, actinomycosis
- Post radiation therapy
- Previous major abdominal surgery or retroperitoneal trauma
- Other e.g. sarcoidosis, amyloidosis, Erdheim–Chester disease (a rare histiocyte disorder)
|
The clinical presentation of RPF can be very subtle, and indeed is often asymptomatic or with nonspecific constitutional symptoms, such as low-grade fever, unexplained weight loss, anorexia and malaise. Because of its insidious nature, diagnosis often only occurs once late complications have developed. Pain is the most common presenting symptom, mainly in the back, flanks and abdomen, and when the ureters are involved can mimic renal colic.3 Lower urinary tract symptoms, including haematuria, frequency, urgency and dysuria, occur less frequently.2 Other urological manifestations, such as testicular pain and swelling, hydrocele and varicocele can develop owing to compression of retroperitoneal gonadal vessels.2,3,6 Encasement of the ureters often leads to obstructive uropathy and, if left untreated, can cause permanent kidney damage. New-onset hypertension (or worsening of pre-existing high blood pressure) may occur owing to impingement of renal vasculature or to hydronephrosis.2 Other vascular complications mainly affect the venous system (most commonly inferior vena cava and iliac veins) with involvement of major arteries being rare except in RPF related to aortic aneurysms. These may result in lower limb swelling, deep vein thrombosis, claudication and even hoarseness of the voice due to recurrent laryngeal nerve compression when the inflammatory mass extends upwards around the thoracic aorta.2,6
There are no specific diagnostic laboratory parameters for RPF, but routine blood tests can provide useful clues. Over half of patients have raised inflammatory markers, such as ESR and CRP, 1,7 and these can be useful in monitoring disease activity and response to treatment. Significant elevation in serum urea and creatinine concentration is not unusual, with 40–50% of patients having renal dysfunction in some studies.7,8 Many patients (around 60%) have a chronic normochromic, normocytic anaemia due to chronic inflammation.1 Given that idiopathic RPF can often occur in association with other autoimmune disorders, screening for autoantibodies such as ANA, ANCA, antismooth muscle antibodies and thyroid autoantibodies may be useful in the initial evaluation.2,6 Serum IgG4 levels should also be measured, although in one series a third of patients had normal serum levels even in the presence of biopsy-proven IgG4 disease.6 Imaging plays an important role in establishing the diagnosis of RPF, and CT or MRI are the initial modalities of choice both to detect fibrosis and to help differentiate between idiopathic and secondary disease.2,3,6 PET-CT scanning can also be useful in assessing disease activity and thus guide treatment, albeit with low specificity for initial diagnosis.2,6,9 Tissue biopsy is not routinely advocated, but should be considered when there is concern over underlying conditions, such as infection, malignancy or possibly when response to steroid therapy is poor.6
Obstructive uropathy due to RPF usually warrants initial surgical or radiological decompression with ureteric stenting or nephrostomy insertion in order to preserve renal function. Management of secondary RPF involves prompt treatment of the underlying cause. Idiopathic RPF often responds to immunosuppression, with corticosteroids being the first-line agents of choice. Oral prednisolone (0.5–1 mg/kg/day) is usually given for approximately 4–6 weeks, with subsequent tapering to a maintenance dose of 5–10 mg/day over a 9–12-month period according to response.2,6 Many patients have a chronic relapsing clinical course, and thus careful monitoring with clinical assessment, measurement of inflammatory markers and serial imaging studies (every 6–12 months) is required during active treatment and surveillance after remission.2,6 Ureteric stents can often be removed after satisfactory regression of inflammatory mass, although some patients require long-term stent changes. Tamoxifen has been proposed as an alternative option in those with contraindications to steroid therapy, although relapse rates were higher than in patients treated with prednisolone in two studies.8,10 Treatment can be challenging in steroid-refractory or relapsing cases, although observational studies have shown promise in the use of alternative immunosuppressants (e.g. methotrexate, azathioprine, cyclophosphamide, ciclosporin).2,6 More recent reports have suggested a role for biological approaches, such as anti-B cell therapy with rituximab or targeting the IL-6 pathway with tocilizumab.2,6 Open ureterolysis or ureteric reconstruction surgery can be considered, although these are often reserved for patients refractory to medical therapy.2
Conclusion
Although RPF is a rare cause of obstructive uropathy, it is important to make an early diagnosis as, left untreated, it can lead to permanent renal dysfunction. Early diagnosis allows the timely relief of obstruction with institution of appropriate medical therapy (in idiopathic disease) or investigation for an underlying cause. Despite its relapsing–remitting nature, idiopathic RPF usually carries a good prognosis; in secondary RPF, outcome is often dictated by the underlying cause.
References
1 van Bommel EF, Jansen I, Hendriksz TR et al. Idiopathic retroperitoneal fibrosis: prospective evaluation of incidence and clinicoradiologic presentation. Medicine (Baltimore) 2009; 88: 193–201.
2 Vaglio A, Maritati F. Idiopathic retroperitoneal fibrosis. J Am Soc Nephrol 2016; 27: 1880–9.
3 Tzou M, Gazeley DJ, Mason PJ. Retroperitoneal fibrosis. Vasc Med 2014; 19: 407–14.
4 Fujimori N, Ito T, Igarashi H et al. Retroperitoneal fibrosis associated with immunoglobulin G4-related disease. World J Gastroenterol 2013; 19: 35–41.
5 Khosroshahi A, Carruthers MN, Stone JH et al. Rethinking Ormond’s disease: “idiopathic” retroperitoneal fibrosis in the era of IgG4-related disease. Medicine (Baltimore) 2013; 92: 82–91.
6 Urban ML, Palmisano A, Nicastro M et al. Idiopathic and secondary forms of retroperitoneal fibrosis: a diagnostic approach. Rev Med Intern 2015; 36: 15–21.
7 Kermani TA, Crowson CS, Achenbach SJ et al. Idiopathic retroperitoneal fibrosis: a retrospective review of clinical presentation, treatment, and outcomes. Mayo Clin Proc 2011; 86: 297–303.
8 Vaglio A, Palmisano A, Alberici F et al. Prednisone versus tamoxifen in patients with idiopathic retroperitoneal fibrosis: an open-label randomised controlled trial. Lancet 2011; 378: 338–46.
9 Vaglio A, Versari A, Fraternali A et al. (18)F-fluorodeoxyglucose positron emission tomography in the diagnosis and followup of idiopathic retroperitoneal fibrosis. Arthritis Rheum 2005; 53: 122–5.
10 Brandt AS, Kamper L, Kukuk S et al. Tamoxifen monotherapy in the treatment of retroperitoneal fibrosis. Urol Int 2014; 93: 320–5.
