Introduction
Transient loss of consciousness (TLoC) affects up to half of the UK population at least once during their lifetime and commonly presents to physicians at all levels.1 We need to be able to recognise and manage the various causes of TLoC appropriately. Ictal asystole, defined as an R–R interval of >3–4 s,2 is a rarer cause of TLoC that is not well known amongst physicians. Therefore, although this is a treatable condition, the diagnosis is often delayed or missed, which may result in significant harm.3 It is important to increase awareness and education around ictal asystole.
Case presentation
Patient one was an otherwise healthy 57-year-old male with a history of sudden and intense episodes of déjà vu followed by TLoC. He would then make a rapid and complete recovery within seconds. There were no abnormal limb movements or tongue biting. The déjà vu was described as a brief (lasting seconds), stereotyped, intensely familiar feeling of detachment. There were six episodes over 2 years, each with no warning or provocation. He had a normal clinical examination, laboratory evaluation and brain MRI. Electroencephalography (EEG) was not performed. An initial diagnosis of vasovagal syncope was made in the cardiology clinic, following a normal 12-lead electrocardiogram and 24-hour Holter monitor, and he was discharged from follow up. However, as the symptoms persisted, the general practitioner referred him to neurology. An alternative diagnosis of focal epilepsy with ictal asystole was considered in the neurology clinic, given the prodrome of déjà vu symptoms. Levetiracetam was recommended and he was referred back to cardiology with a request for more prolonged monitoring. An implantable cardiac monitor was inserted. During a subsequent episode of déjà vu and TLoC, the implantable cardiac monitor captured marked sinus bradycardia at 10 beats per minute, progressing to periods of ventricular standstill. The patient underwent dual-chamber permanent pacemaker (PPM) insertion, which abolished TLoC. However, in the absence of TLoC, déjà vu became more frequent, intense and prolonged, at which point it became apparent that levetiracetam had not been commenced as originally suggested. The déjà vu resolved after this antiepileptic drug (AED) was prescribed and he remained seizure free for several months. He then experienced several secondary generalised tonic–clonic seizures (GTCSs) and was switched to carbamazepine, on which he has continued to remain seizure free.
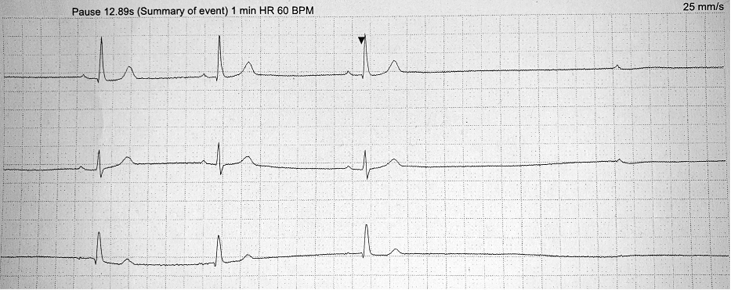
Patient two was a 45-year-old female with type 1 diabetes. She presented to the first seizure clinic following her first GTCS. On questioning, she provided an 8-year history of episodes of déjà vu, describing dreamlike experiences that were brief (lasting seconds), stereotyped and intensely familiar. They occurred once every few months and usually resolved spontaneously. However, on some occasions, the déjà vu was followed by TLoC with rapid and complete recovery within seconds. On all occasions, her blood sugar was within normal limits. A clinical diagnosis of focal epilepsy was made and lamotrigine commenced. EEG and MRI head were normal. The history of TLoC and rapid recovery that followed some of her déjà vu episodes prompted a search for cardiac arrhythmias in association with the focal seizures. A 48-hour Holter monitor was arranged. This captured an episode of déjà vu and TLoC during which cardiac asystole was identified as sinus arrest and ventricular standstill with a 13-s pause (Figure 1). She underwent dual-chamber PPM insertion and lamotrigine was stopped. She then re-presented with déjà vu followed by a GTCS despite a functioning PPM. Lamotrigine was restarted and she has since remained seizure free at 12 months.
Figure 1 A three-lead Holter monitor trace demonstrating the onset of cardiac asystole during a stereotyped episode of intense déjà vu followed by transient loss of consciousness (TLoC) in patient two. This period of ventricular standstill carried on for 12.89 s before normal sinus rhythm resumed. BPM: beats per minute; HR: heart rate
Discussion
Diagnostic challenges
A rapid recovery following TLoC is usually consistent with a diagnosis of syncope,4,5 which was the presumed diagnosis for patient one initially. However, careful probing of the history revealed a stereotyped prodrome of brief déjà vu symptoms, and these should alert physicians that the patient is likely to be, instead, having focal seizures that are being complicated by ictal asystole. During the asystole, the patient loses consciousness as they would in syncope, hence the potential for diagnostic confusion. However, the underlying aetiology is epilepsy – which should not be missed and requires treatment. Indeed, despite levetiracetam and a functioning PPM, patient one went on to have GTCSs and required a change to carbamazepine. Patient two’s presentation with a déjà vu episodes and a GTCS seemed to indicate an uncomplicated diagnosis of focal epilepsy with secondary GTCSs. However, careful probing of the history revealed episodes of syncopal-sounding TLoC following the déjà vu events at times, and physicians should be aware that these raise the possibility that the patient is having their focal epilepsy complicated by ictal asystole. It is important to recognise this feature in the history as management may include not only AEDs, but also cardiology input for pacemaker consideration. In Box 1, we summarise the key features of a clinical history that may be suggestive of ictal asystole. Such patients warrant simultaneous referral to both neurology and cardiology with the query of ictal asystole specifically raised.
Box 1 Red flag features in the clinical history that may be suggestive of ictal asystole
|
Red flags for when diagnosing syncope:
- A prodrome suggestive of seizures:
- Rising epigastric sensation
- Déjà vu or jamais vu
- Dreamlike state
- Abnormal taste/smell
- Episodes not previously appreciated as seizures, e.g. nocturnal events (waking up with tongue trauma, myalgia, confusion, lethargy)
- Risk factors for epilepsy (structural brain lesions, febrile convulsions, developmental/intellectual delay, family history of epilepsy)
Red flags for when diagnosing seizures:
- Episodes are interrupted or followed by syncope, i.e. an abrupt loss of consciousness with rapid and spontaneous recovery (usually within minutes)
- Family history of a sudden unexplained death
|
Ictal asystole is a recognised complication of focal epilepsy, with a point prevalence of 0.32%.2 The origin of seizures is temporal in 90% of cases.2 When diagnosing temporal lobe epilepsy (TLE), interictal EEG showing focal epileptiform discharges may help further characterise seizures, but this investigation is normal in 71% of cases.6 Therefore, the diagnosis remains one that should be made on clinical grounds.6,7 More than 80% of patients with TLE describe an aura (focal seizure) with experiential and viscerosensory symptoms, such as the déjà vu described by our patients.6 Such a history is often sufficient to make a clinical diagnosis of focal epilepsy.6,8
Ictal asystole presents as TLoC during a focal seizure. The asystole is a direct consequence of seizure activity simulating the central autonomic network.2,3 The mean duration of ventricular standstill is 20 s. During this, there is cerebral anoxia-ischemia and this results in termination of the seizure before it can progress further, and, therefore, termination of the asystole. In this manner, ictal asystole is normally self-limiting. However, a minority continue into postictal asystole, which is fatal in 54% of cases.2 Postictal asystole is typically associated with secondary GTCSs and prolonged postictal apnoea that leads to cardiac arrest requiring resuscitation, and probably explains a proportion of sudden unexpected death in epilepsy (SUDEP).2 Ictal asystole has a variable expression and, therefore, does not occur during every seizure. Abolishing the asystole with a pacemaker removes the asystolic seizure self-termination mechanism, unmasking worsened seizures,3 as was seen in both of our patients after pacemaker insertion.
Management challenges
The National Institute for Health and Care Excellence (NICE) recommends that patients presenting with TLoC and prodromal déjà vu or jamais vu should be referred for an epilepsy specialist review within 2 weeks.1 There are no guideline-directed therapies for ictal asystole.9 This is likely owing to a lack of randomised trials. Whilst there is some literature suggesting that optimisation of AEDs alone may be effective in preventing ictal asystole, cardiac pacemakers help prevent the morbidity associated with falls secondary to asystole when a seizure does occur.9 This is important as only 60% of patients with newly diagnosed epilepsy achieve seizure freedom on their first or second AED.10 In the seizure-free group, there is still a risk of seizure recurrence (and hence asystole) in the context of, for example, missed AEDs or vomiting. Therefore, as it is not possible to guarantee patients seizure freedom using AEDs, we argue that patients should not be exposed to the predictable risk of harm in relation to loss of consciousness or a fall from the ictal asystole. Furthermore, a causal role for ictal asystole in SUDEP, although debated, is yet to be excluded.2,3,11 Cardiology guidelines recommend insertion of a PPM in symptomatic sinus bradycardia.4,12 There is no established minimum heart rate or pause duration at which PPM insertion is recommended; only the occurrence of symptoms is important.4,12 Therefore, notwithstanding the possibility of AEDs alone being sufficient to prevent further seizures in some patients, once a patient has presented with the symptom of TLoC from ictal asystole, the management strategy seeming to most closely follow these guidelines would appear to be with PPM insertion. These issues should be discussed openly with patients in helping them come to a decision on their therapy. In our centre, we prefer a cautious management approach involving both AEDs and a PPM.
Patients with ictal asystole are often seen by neurologists, cardiologists or both, but almost always separately. Information may go backwards and forwards between the two teams, but patients are at risk if that information sharing is incomplete. For one of our patients, that miscommunication resulted in a recommended AED never starting. For the other, it resulted in their AED being stopped. A joint cardiology and neurology clinic may be the best way forward to manage patients with ictal asystole. This may also assist in the management of some cardiac arrhythmias that can mimic epilepsy, such as long QT syndrome.13 In the absence of joint clinics, physicians need to communicate more closely about these patients.
Conclusion
Careful attention should be paid to symptoms suggestive of focal seizures (e.g. déjà vu) in patients presenting with what otherwise sounds like syncope – they may have epilepsy presenting with ictal asystole. Equally important is paying careful attention to symptoms of syncope in what is otherwise sounds like a typical history of seizures – these patients may also have ictal asystole. Both AED treatment and cardiac investigations should be simultaneously arranged for patients suspected of ictal asystole. We encourage a cautious approach with AEDs and pacemaker insertion when managing patients with ictal asystole. The continuation of AEDs following pacemaker insertion is vital, as treating the cardiac arrhythmia may unmask seizures. Safe management of ictal asystole requires close communication between cardiology, neurology and admitting physicians. Any plans for commencing AEDs and inserting a pacemaker should be shared explicitly between teams. The patient’s perspective provided in Appendix A helps to contextualise these recommendations.
Acknowledgements
We thank the patients for kindly providing us with their support and written consent for publication of this material.
Online Supplementary Material
Appendix A is available with the online version of this paper, which can be accessed at https://www.rcpe.ac.uk/sites/default/files/jrcpe_49_2_mbizvo_suppl.pdf.
References
1 National Institute for Health and Care Excellence. Clinical guideline [CG109]: Transient loss of consciousness (‘blackouts’) in over 16s. 2010. https://www.nice.org.uk/guidance/cg109 (accessed 08/04/19).
2 van der Lende M, Surges R, Sander JW et al. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry 2016; 87: 69–74.
3 Benditt DG, van Dijk G, Thijs RD. Ictal asystole: life-threatening vagal storm or a benign seizure self-termination mechanism? Circ Arrhythm Electrophysiol 2015; 8: 11–4.
4 Kusumoto FM, Schoenfeld MH, Barrett C et al. 2018 ACC/AHA/hrs guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay. Circulation 2018; CIR0000000000000628.
5 Shen WK, Sheldon RS, Benditt DG et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017; 136: e60–122.
6 Scheffer IE, Berkovic S, Capovilla G et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017; 58: 512–21.
7 National Institute for Health and Care Excellence. Clinical guideline [CG137]: Epilepsies: diagnosis and management. 2012. https://www.nice.org.uk/guidance/cg137 (accessed 08/04/19).
8 Acharya V, Acharya J, Luders H. Olfactory epileptic auras. Neurology 1998; 51: 56–61.
9 Kepez A, Erdogan O. Arrhythmogenic epilepsy and pacing need: a matter of controversy. World J Clin Cases 2015; 3: 872–5.
10 Chen Z, Brodie MJ, Liew D et al. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol 2018; 75: 279–86.
11 So NK, Sperling MR. Ictal asystole and SUDEP. Neurology 2007; 69: 423–4.
12 National Institute for Health and Care Excellence. NICE Pathways: Heart rhythm conditions overview. 2018. https://pathways.nice.org.uk/pathways/heart-rhythm-conditions (accessed 08/04/19).
13 Galtrey CM, Levee V, Arevalo J et al. Long QT syndrome masquerading as epilepsy. Pract Neurol 2019; 19: 56–61.
