Introduction
The prognosis and prospects for people newly diagnosed with HIV in the UK are excellent; with timely and effective treatment, people living with HIV (PLWH) have a normal life expectancy1 and are not at risk of transmitting the virus sexually.2–5 In 2018, it was estimated that 93% of PLWH in the UK knew their HIV status, 97% of those diagnosed were on treatment, and that 97% of those on treatment were virologically suppressed,6 far surpassing the UNAIDS 90-90-90 treatment target for 2020.
Unfortunately, the proportion of new HIV diagnoses in the UK that are made at a late stage of infection (CD4 cell count <350 cells/mm3 within 3 months of diagnosis) remains high, meaning that many PLWH are not able to fully benefit from these advances. In 2018, 43% of PLWH were diagnosed late, compared to 52% in 2009. Late diagnosis is associated with an increased risk of infectious and inflammatory consequences, and is the single most important predictor of HIV-related mortality and morbidity, with individuals who present or who are diagnosed late being ten times more likely to die in the year following diagnosis than those diagnosed at an earlier stage.7 The timely diagnosis of HIV is, therefore, a public health priority and screening and testing for HIV in at-risk populations is vital to allow this to happen.
National guidelines suggest that HIV testing should be offered to all general medical admissions areas with high or extremely high prevalence of HIV (diagnosed HIV prevalence >2 per 1,000 people aged 15–59 years).8–10 In addition, it is well established that HIV testing should be offered to all patients presenting to hospital with conditions considered AIDS-defining in PLWH, and those in which the undiagnosed prevalence of HIV exceeds, or is likely to exceed, 0.1%, i.e., HIV indicator conditions. Individuals with epidemiological risk factors for HIV acquisition should also be offered screening.
Screening for HIV in indicator conditions, regardless of any demographic or behavioural risk assessment, has been shown to decrease the probability of a late diagnosis11 and is cost-effective when using the threshold of 0.1% HIV prevalence.12,13 Despite the compelling evidence for an indicator approach to screening for HIV, and its endorsement by national bodies,8,10,14 compliance with this screening approach is poor.15,16
In 2009, we conducted a survey of knowledge, attitudes and practice regarding HIV testing among non-HIV specialist hospital physicians that identified limited awareness of specific guidance on HIV testing and indicator conditions, low self-reported rates of HIV testing in routine clinical practice and aberrant perceptions of the acceptability of routine testing among the patient population.17
We designed this follow-up study to ascertain whether awareness of indications for HIV testing amongst non-HIV specialist physicians had improved in the decade since our last survey, and following the release of further national guidance. We also sought to identify any ongoing perceived barriers to offering HIV tests in secondary care, with the aim of identifying potential targets for future educational initiatives.
Methods
Study sites and target population
We conducted a multicentre study across three NHS hospital trusts: NHS Lothian, The Newcastle-upon-Tyne Hospitals (NUTH) NHS Foundation Trust, and Northumbria Healthcare NHS Foundation Trust (NHCT). Surveys were distributed to consultants, specialist registrars and senior grade doctors working in 15 major hospital specialties (Box 1). These specialties were identified by a panel of Infectious Diseases physicians as being likely to encounter patients presenting with clinical indicator conditions on a regular basis, either through specialty care or contribution to the acute medical take.
Box 1 Target hospital specialties
- Critical Care and Anaesthetics
- Acute and General Internal Medicine
- Obstetrics and Gynaecology
- Gastroenterology/Hepatology
- Neurology
- Oncology
- Respiratory Medicine
- Renal Medicine
- Rheumatology
- Care of the Elderly and Stroke Medicine
- Dermatology
- Haematology
- Cardiology
- Diabetes and Endocrinology
- Ophthalmology
At the time this survey was conducted, the overall diagnosed HIV prevalence rate per 1,000 of the population aged 15–59 years was 2.35 in NHS Lothian18 and 1.17 in NE England.19 The prevalence varies between areas in these regions; in Newcastle-upon-Tyne the prevalence is around 1.88–2.29/1,000, whereas in Northumberland the prevalence is lower at 0.60–0.87/1,000.19
Questionnaire design
We developed an electronic nine-item questionnaire (Supplementary data) which was administered using the SurveyMonkeyTM online cloud-based survey platform. The questionnaire was designed to assess knowledge, attitudes and practice amongst non-HIV specialist physicians with regards to HIV testing in secondary care. To allow for comparison between the present study and previous results from 2009, the questionnaire was based on a template used previously,17 but updated to reflect changes in terminology and national guidelines (Table S1). As previously, attitudes were assessed by asking respondents to indicate any perceived barriers to testing, with space for free-text comments provided. Current practice was assessed by asking respondents to quantify the number of HIV tests they had requested in the year prior to completing the questionnaire.
Delivery of survey
In advance of the survey being disseminated, an individual working within each hospital specialty was approached and asked to act as a lead contact for the study. These individuals agreed to distribute a short email to senior doctors working within their specialty on a set date, and to follow this up with an email reminder after 2 weeks.
Analysis
Survey data were exported from SurveyMonkey into Microsoft Excel; statistical analyses were performed using IBM SPSS Statistics 25. Categorical variables were compared using Pearson chi-squared test; a p-value of <0.05 was accepted as good evidence of statistical association. Overall frequencies of responses to nominal categories were summarised, differences and associations between groups of respondents according to site, specialty and grade were assessed, and qualitative differences in overall frequency of responses to nominal categories between 2009 and 2019 were described.
Results
Participants
In Lothian, 162 responses were received from a total of 729 initial survey requests (22.2%). A further 176 responses were received from NUTH, and 86 from NHCT. Data on overall response rates from sites outwith NHS Lothian were not available.
Responses from physicians and specialties not on the target list but erroneously included were removed, leaving 354 responses available for further analysis. The majority of responses were provided by NHS Lothian (n = 154, 43.5%), followed by NUTH (n = 123, 34.7%) and NHCT (n = 77, 21.8%).
The majority of respondents were consultant grade (n = 228, 64.4%), followed by specialist trainees (n = 105, 29.7%) and specialty doctors (n = 21, 5.9%). The frequency of responses according to grade did not differ significantly between sites (p = 0.07).
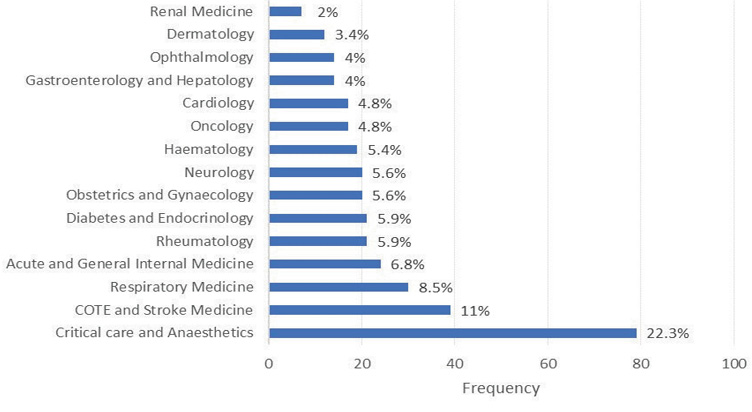
Responses were received from all targeted specialties (Figure 1); the greatest frequency of responses came from Critical Care and Anaesthetics (n = 79, 22.3%), followed by Care of the Elderly and Stroke Medicine (n = 39, 11%). The response rate according to specialty varied significantly between sites (p < 0.0001).
Figure 1 Overall responses according to specialty
Knowledge and practice
The majority of respondents had requested less than 5 HIV tests in the preceding year (n = 175, 49.4%). A smaller proportion had requested between 5 and 10 HIV tests (n = 62, 17.5%) and around a third of study participants reported requesting greater than 10 HIV tests (n = 117, 33.1%).
Most respondents were aware that HIV testing no longer required pre-test counselling (n = 293, 82.8%). However, 21 respondents were not sure (5.9%) and 40 clinicians believed that it was still required (11.3%).
A minority of respondents believed that any HIV tests need to be declared when applying for life insurance (n = 20, 5.6%) and a further 82 study participants were not sure (23.2%). However, most respondents were aware that this is not necessary (n = 252, 71.2%).
There were no significant differences in the number of HIV tests requested, or beliefs about the requirement for pre-test counselling or the need to declare to an insurance company between sites or between grades of respondent.
Testing in non-AIDS defining indicator conditions
The conditions most frequently identified as indicators for HIV testing were unexplained lymphadenopathy (n = 268, 75.7%), pyrexia of unknown origin (n = 252, 71.2%), unexplained weight loss (n = 232, 65.5%) and sexually transmitted infections (n = 232, 65.5%) (Figure 2). The least frequently recognised condition was lung cancer (n = 53, 15.0%), followed by seborrhoeic dermatitis (n = 66, 18.6%), severe psoriasis (n = 72, 20.3%) and shingles (n = 84, 23.7%). Nearly 5% of respondents did not recognise the need to offer an HIV test in any of the selected indicator conditions (n = 16, 4.5%). Only 13% of clinicians recognised the need to test for HIV in all of the suggested indicator conditions (n = 46), and there was no significant difference according to grade of respondent (consultant 13.2%, specialty trainee 13.3%, staff grade 9.5%; p = 0.887).
Figure 2 Proportion of respondents who would offer an HIV test according to the selected indicator condition, arranged according to frequency. AIN: anal intraepithelial neoplasia; CIN: cervical intraepithelial neoplasia; PUO: pyrexia of unknown origin
In general, physicians were better at recognising the indicator conditions relevant to their specialty. For instance, 96.7% of Respiratory physicians reported that they would offer an HIV test to patients presenting with tuberculosis (compared to an average of 51.9% for all other specialties), and 63.3% would offer an HIV test to patients presenting with a community-acquired pneumonia (compared to an average of 26.2% for all other specialties (Table S2).
Neurology had the highest proportion of specialist physicians who reported that they would offer an HIV test in all of the indicator conditions (30%), followed by Acute and General Medicine (25%). Obstetrics and Gynaecology had the highest proportion of clinicians who would not test for HIV in any of the studied indicator conditions (15%), followed by Critical Care and Anaesthetics (12.7%).
Attitudes
The most frequently identified barrier to offering an HIV test in respondents’ daily practice was the perception of working with a low-risk patient population (n = 119, 33.6%). Nearly a quarter of study participants also cited a lack of training and education around offering HIV tests (n = 83, 23.4%). A fifth of respondents identified concerns regarding lack of patient acceptance (n = 75, 21.2%). Few clinicians identified institutional costs (n = 6, 1.7%), informing a patient of an HIV diagnosis (n = 22, 6.2%) or concerns regarding confidentiality (n = 12, 3.4%) as barriers. Over a third of clinicians did not identify any barriers to offering an HIV test (n = 128, 36.2%).
Respondents from NUTH (n = 48, 39.0%) and NHCT (n = 32, 41.6%) more frequently identified working with a low-risk patient population as a barrier to offering an HIV test than respondents from NHS Lothian (n = 39, 25.3%), although this difference did not reach statistical significance (p = 0.14). Responses were otherwise similar between sites.
Comparison over time
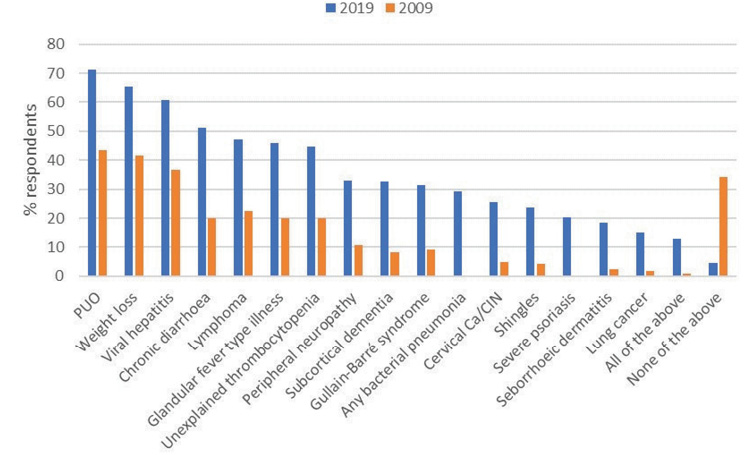
Between 2009 and 2019, the proportion of respondents who would offer an HIV test increased for all indicator conditions, by an average of 23.1% (standard deviation 4.6) (Figure 3). In 2009, only 0.8% (n = 1/120) of survey recipients responded that they would test for HIV in all indicator conditions, compared to 12.9% (n = 46/354) in 2019 (p < 0.001). In a more strictly defined comparison including only those sites and specialties sampled in the first survey, the number of physicians who responded that they would test in all of the selected indicator conditions rose from 0.8% in 2009 to 16.5% (n = 15/91) in 2019.
Figure 3 Proportion of respondents who would offer an HIV test according to the selected indicator condition; a comparison over time. CIN: cervical intraepithelial neoplasia; PUO: pyrexia of unknown origin
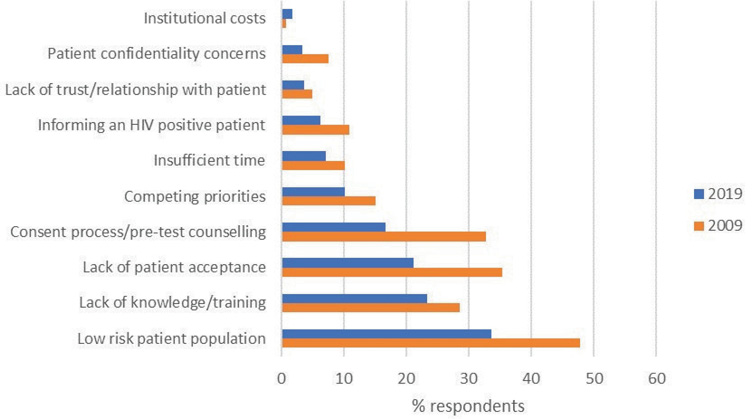
In parallel with an apparent increased awareness of clinical indications for HIV testing, the frequencies of citing barriers to offering HIV tests appeared to decrease (Figure 4). The perception of working with a low-risk patient population remained the most frequently identified barrier to offering an HIV test in 2019, although the proportion of respondents who identified this as a deterrent significantly decreased over time (47.9% of respondents in 2009, compared with 33.6% in 2019; p = 0.007). The most marked changes over time were noted in responses to questions regarding the consent process and the need for pre-test counselling (32.5% in 2009 compared to 16.7% in 2019; p < 0.001), and lack of patient acceptance (35.8% in 2009, 21.2% in 2019; p = 0.001). Just over a third of patients in 2019 did not perceive any barriers to offering an HIV test (n = 128, 36.2%).
Figure 4 Identified barriers to offering an HIV test; a comparison over time
Discussion
In this longitudinal, multicentre study we have demonstrated that awareness of indications for HIV testing has improved over time. However, there was wide variation in responses between specialties and only a minority of respondents recognised the need to test for HIV in all indicator conditions.
The proportion of respondents who recognised the need to offer an HIV test increased for all indicator conditions over a 9-year period, indicating an increased awareness of HIV testing guidelines. However, only a third of clinicians had requested more than 10 HIV tests in the last year, indicating that this awareness has not yet translated into a change in practice.
There was variability in the awareness of testing indications amongst the specialties surveyed. Clinicians working in Acute and General Internal Medicine and Neurology were most likely to offer an HIV test in all of the indicator conditions. These specialties have been targets for educational input and their increased awareness over time of HIV testing guidelines may reflect the success and therefore value of such initiatives.20,21 Additionally, specialties were more likely to recognise the need to offer an HIV test in indicator conditions seen more frequently in their clinical practice. Obstetrics and Gynaecology and Critical Care and Anaesthetics were the specialties least likely to offer an HIV test in any of the selected indicator conditions, which may reflect lack of exposure to the acute unselected medical take.
Although lung cancer was the least frequently identified HIV indicator condition in our study, dermatological conditions, including shingles, seborrhoeic dermatitis and psoriasis, were also poorly recognised. Dermatological conditions are commonly identified as missed indicators in patients diagnosed at a late stage of HIV infection22 and our findings support a targeted educational initiative.
The frequency with which barriers to offering an HIV test were identified appears to have reduced over time, although nearly two-thirds of respondents perceived ongoing obstacles. The most frequently identified barrier to offering an HIV test was the perception of working with a low-risk patient population. This has also been identified as a patient factor influencing the uptake of HIV testing.23
Over one-fifth of respondents identified lack of knowledge and training as barriers to offering testing. These findings are consistent with previous survey results.24,25 A recently published systematic review demonstrated that intrapersonal barriers strongly influenced the routine offer of an HIV test;26 such barriers often reflect assumptions about patients’ perspectives and issues relating to the consent process, as demonstrated in our cohort. However, the proportion of respondents in our study who identified the consent process as a barrier reduced significantly over time. This indicates an awareness of the more recent guidance available from the British HIV Association (BHIVA) and the National Institute for Health and Care Excellence (NICE); individuals should be made aware that they are being tested and that it is voluntary, however, lengthy pre-test discussion is not required.8–10
Although the proportion of respondents who identified lack of patient acceptance as a barrier to HIV testing significantly decreased over time, a fifth of clinicians still identified this as a concern. The offer of an HIV test has consistently been shown to be acceptable to patients in both primary and secondary care,27,28 and the limiting factor is often failure of the healthcare provider to offer an HIV test. Healthcare providers frequently cite lack of confidence in offering an HIV test, the stigma and exceptionalism associated with HIV testing, lack of time to counsel patients and uncertainty with dealing with results as barriers to testing.29 These are additional areas that could readily be addressed through targeted education.
Our results indicate that although testing rates have improved, further action is required to bring about continued change and improvement in the practice of non-HIV specialist physicians. Behavioural change requires interventions that increase opportunity and capability that in turn give motivation for change.30 We know that physicians have opportunity for testing as they encounter patients with indicator conditions in their daily work. Therefore, improving capability through targeted education of healthcare providers is the main potential intervention to promote change and indeed has been shown to improve testing rates for HIV.20,31,32 However, ‘one-off’ training sessions on HIV testing are likely to require further interventions such as designated HIV testing liaison health advisors33 and/or computer-based prompting to provide lasting benefits.34,35 As undiagnosed individuals may present to a variety of settings, improving local screening processes requires education and engagement of health and social care workers throughout primary and secondary care facilities.36 It is important that these factors are also considered when developing educational strategies.
This study has several important limitations. Whilst electronic surveys have the advantages of quick responses, rapid data collation and access to a large target population, lower response rates are well recognised.37 We sought to optimise response rates by keeping the questionnaire short, indicating the time required for completion in the cover letter, embedding the survey link within the email, and involving a lead individual within each specialty.38 Despite these measures the response rate where measured was low, but not dissimilar to those described in other published surveys, and would be considered typical for an online survey in which there is no prior relationship with recipients.39 Receiving over 300 responses in this context is relatively large sample size and as such we have been able to report a breadth of practice.
Additionally, self-selection bias is inherent in studies of survey responses; it is possible that respondents had a greater pre-existing degree of interest in the topic and findings may not be representative of the larger medical body.
We amended the terminology regarding the HIV indicator conditions between surveys, to ensure that our wording was consistent with current HIV testing guidance.10 For instance, ‘psoriasis’ was replaced by ‘severe psoriasis’. It is possible that this may have influenced the survey responses by increasing the likelihood that these conditions were selected as indicators, and should be taken into consideration when interpreting changes over time.
Previous audits of HIV testing in hospital departments have shown that doctors working in hospital settings are not always aware of testing guidelines.40 Unfortunately, due to a technical error with the online survey responses we were unable to assess physician’s knowledge of established NICE and BHIVA guidelines. Additionally, we did not enquire as to whether the patient’s age influenced the decision to offer an HIV test; older age increases the risk of late HIV diagnosis41 and this should be included in any related future work.
The strengths of this study are the longitudinal, multicentre design. As far as we are aware, this is the first study to address the opinions of non-HIV specialist physicians towards HIV testing in areas of low HIV prevalence over time. This study provides valuable insight into the areas where further educational initiatives should be targeted and highlights the challenges of professional behaviour change.
In summary, our study demonstrates that although awareness of HIV testing indications and rates of HIV testing have improved over time, practice in North-East England and South-East Scotland remains suboptimal and there is an ongoing need for targeted and sustained educational initiatives. 
Online Supplementary Material
Supplmentary data are available with the online version of this paper, which can be accessed at https://www.rcpe.ac.uk/journal.
References
1 May MT, Gompels M, Delpech V et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014; 28: 1193–202.
2 Del Romero J, Castilla J, Hernando V et al. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ 2010; 340: c2205.
3 Castilla J, Del Romero J, Hernando V et al. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr 2005; 40: 96–101.
4 Cohen MS, Chen YQ, McCauley M et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375: 830–9.
5 Rodger AJ, Cambiano V, Bruun T et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316: 171–81.
6 O’Halloran C, Sun S, Nash S et al. HIV in the United Kingdom: towards zero 2030. 2019 report. London: Public Health England; December 2019.
7 Sobrino-Vegas P, Moreno S, Rubio R et al. Impact of late presentation of HIV infection on short-, mid- and long-term mortality and causes of death in a multicenter national cohort: 2004–2013. J Infect 2016; 72: 587–96.
8 BHIVA. Adult HIV Testing Guidelines 2020. Letchworth: British HIV Association; 2020.
9 BHIVA. UK National Guidelines for HIV Testing 2008. Letchworth: British HIV Association; September 2008.
10 Excellence NIfHaC. HIV Testing: Increasing Uptake Among People Who May Have Undiagnosed HIV. London: NICE; December 2016.
11 Scognamiglio P, Chiaradia G, De Carli G et al. The potential impact of routine testing of individuals with HIV indicator diseases in order to prevent late HIV diagnosis. BMC Infect Dis 2013; 13: 473.
12 Graves N, Walker DG, McDonald AM et al. Would universal antenatal screening for HIV infection be cost-effective in a setting of very low prevalence? Modelling the data for Australia. J Infect Dis 2004; 190: 166–74.
13 Sullivan AK, Raben D, Reekie J et al. Feasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study). PLoS One 2013; 8: e52845.
14 HIV in Europe. HIV Indicator Conditions: Guidance for Implementing HIV Testing in Adults in Health Care Settings. Copenhagen: HIV in Europe; 2012.
15 Acquah RR, Baggott A, McGoldrick C et al. HIV testing in Lanarkshire. J R Coll Physicians Edinb 2014; 44: 278–82.
16 Raben D, Mocroft A, Rayment M et al. Auditing HIV testing rates across Europe: results from the HIDES 2 Study. PLoS One 2015; 10: e0140845.
17 Hunter E, Perry M, Leen C et al. HIV testing: getting the message across–a survey of knowledge, attitudes and practice among non-HIV specialist physicians. Postgrad Med J 2012; 88: 59–65.
18 Public Health Scotland. HIV in Scotland: update to 31 December 2019. Edinburgh: Public Health Scotland; 2019.
19 Public Health England. Public Health Profiles 2020. https: //fingertips.phe.org.uk/search/HIV#page/0/gid/1/pat/6/par/E12000001/ati/102/are/E06000047/cid/4/page-options/ovw-do-0 (accessed 29/11/20).
20 Sokhi DS, Oxenham C, Coates R et al. Four-stage audit demonstrating increased uptake of HIV testing in acute neurology admissions using staged practical interventions. PLoS One 2015; 10: e0134574.
21 Onen BL, Sinha A, Ratnaike T et al. HIV testing on the medical admissions unit: P137. HIV Med 2015; 16: 55.
22 Lin YD, Garner SE, Lau JSY et al. Prevalence of HIV indicator conditions in late presenting patients with HIV: a missed opportunity for diagnosis? QJM 2019; 112: 17–21.
23 Deblonde J, De Koker P, Hamers FF et al. Barriers to HIV testing in Europe: a systematic review. Eur J Public Health 2010; 20: 422–32.
24 Partridge DG, Collini P, McKendrick MW. HIV testing: the boundaries. A survey of HIV testing practices and barriers to more widespread testing in a British teaching hospital. Int J STD AIDS 2009; 20: 427–8.
25 Burke RC, Sepkowitz KA, Bernstein KT et al. Why don’t physicians test for HIV? A review of the US literature. AIDS 2007; 21: 1617–24.
26 Bagchi AD, Davis T. Clinician barriers and facilitators to routine HIV testing: a systematic review of the literature. J Int Assoc Provid AIDS Care 2020; 19: 2325958220936014.
27 Palfreeman A, Nyatsanza F, Farn H et al. HIV testing for acute medical admissions: evaluation of a pilot study in Leicester, England. Sex Transm Infect 2013; 89: 308–10.
28 Ashby J, Braithewaite B, Walsh J et al. HIV testing uptake and acceptability in an inner city polyclinic. AIDS Care 2012; 24: 905–9.
29 Thornton AC, Rayment M, Elam G et al. Exploring staff attitudes to routine HIV testing in non-traditional settings: a qualitative study in four healthcare facilities. Sex Transm Infect 2012; 88: 601–6.
30 Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011; 6: 42.
31 Freer J, Lascar M, Phiri E. Tailoring HIV testing in a setting of late HIV diagnosis: is the tide turning? Br J Hosp Med (Lond) 2015; 76: 592–5.
32 Martínez Sanz J, Pérez Elías MJ, Muriel A et al. Outcome of an HIV education program for primary care providers: screening and late diagnosis rates. PLoS One 2019; 14: e0218380.
33 Mahendran P, Soni S, Goubet S et al. Testing initiatives increase rates of HIV diagnosis in primary care and community settings: an observational single-centre cohort study. PLoS One 2015; 10: e0124394.
34 Kesten JM, Davies CF, Gompels M et al. Qualitative evaluation of a pilot educational intervention to increase primary care HIV-testing. BMC Fam Pract 2019; 20: 74.
35 van Schaik P, Lorrimer S, Chadwick D. Designing an electronic blood-borne virus risk alert to improve uptake of testing. Int J STD AIDS 2020; 31: 800–7.
36 Lazarus JV, Hoekstra M, Raben D et al. The case for indicator condition-guided HIV screening. HIV Med 2013; 14: 445–8.
37 Jones TL, Baxter MA, Khanduja V. A quick guide to survey research. Ann R Coll Surg Engl 2013; 95: 5–7.
38 McPeake J, Bateson M, O’Neill A. Electronic surveys: how to maximise success. Nurse Res 2014; 21: 24–6.
39 Rice HE, Frush DP, Harker MJ et al. Peer assessment of pediatric surgeons for potential risks of radiation exposure from computed tomography scans. J Pediatr Surg 2007; 42: 1157–64.
40 Gupta ND, Lechelt M. Assessment of the implementation and knowledge of the UK National Guidelines for HIV Testing (2008) in key conditions at a UK district general hospital. Int J STD AIDS 2011; 22: 102–4.
41 Levy I, Maor Y, Mahroum N et al. Missed opportunities for earlier diagnosis of HIV in patients who presented with advanced HIV disease: a retrospective cohort study. BMJ Open 2016; 6: e012721.