Giant cell arteritis (GCA) is a primary systemic vasculitis preferentially affecting the branches of the external carotid artery and the subclavian artery. In the UK it has an annual incidence of 2.2/10,000 in those over the age of 50.1 The superficial temporal artery and the maxillary artery are the terminal branches of the external carotid artery. The involvement of the superficial temporal artery is well documented and even lent its name to the disease.2 Scalp tenderness either related to direct inflammation of the branches of the superficial temporal artery or relative ischaemia of the scalp circulation is well known. Nevertheless, inflammatory changes of this scalp artery have no reason to cause headache, jaw claudication, toothache, visual symptoms or proximal muscle stiffness – symptoms that have been relied upon to create a clinical pattern of GCA. This clinical pattern is important in the recognition of a condition that otherwise has no gold standard diagnostic test. Involvement of the maxillary artery is probably responsible for headache (via the involvement of the middle meningeal artery), jaw claudication (branches to the pterygoids and the masseter), toothache (via the superior and inferior alveolar arteries) and perhaps even visual involvement (via the various anastomotic links with the ophthalmic artery). However, there has only been one study so far of involvement and visualisation of the maxillary artery using positron emission tomography.3 In our centre, we have established a validated GCA ultrasonography service.4 In this paper, we have used the definitions for ultrasonographic abnormalities in GCA5 and applied them to the technique of visualising the maxillary artery as described by Lepic et al.6 Using this technique, we describe two cases where the maxillary artery was visualised and found to have changes diagnostic of GCA.
Case presentation
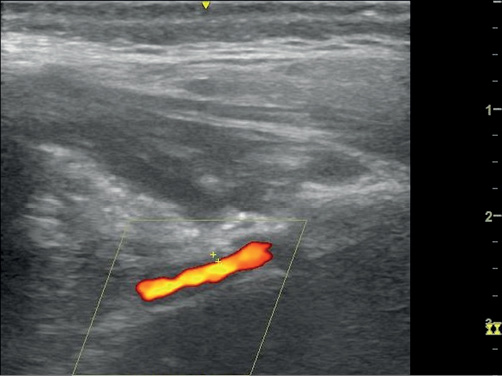
A 78-year-old man presented to the eye casualty with a two-week history of headache, night sweats and diplopia. On examination, he had a thickened left temporal artery and a weakness of the left superior oblique suggesting a fourth nerve palsy. The haemoglobin was 130 g/L and C-reactive protein (CRP) was 71 mg/L. A clinical diagnosis of GCA was made. He was started on the Norwich prednisolone regimen at a dose of 50 mg daily.7 Diagnostic ultrasonography was performed three days later. Both superficial temporal arteries had non-compressible concentric hypoechoic thickening (halo) of the intima-media, diagnostic of GCA.5 The maximum thickness in the superficial temporal artery was 0.4 mm bilaterally. We also imaged the maxillary artery. A halo of 0.7 mm was observed on the right and 0.8 mm on the left (Figure 1). On review five weeks later, he was asymptomatic and his CRP had fallen to 4 mg/L.
Figure 1 Longitudinal (left) and transverse (right) view of the maxillary artery demonstrating hypoechoic concentric thickening measuring 0.8 mm (left), 0.5 mm (right). Images taken with L4-12t probe on a GE LOGIQ e ultrasonography machine. B mode frequency 8.0 MHz; colour frequency 4.2 MHz; pulse repetition frequency 1.9.
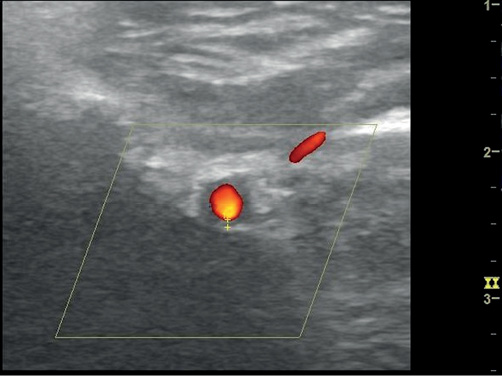
An 80-year-old woman was referred to the rheumatology clinic with a six-month history of weight loss and anorexia. She had a previous history of bowel cancer. Her CRP was >120 mg/L consistently over the previous six months. Investigations geared towards raised inflammatory markers and included a computed tomography (CT) scan of chest, abdomen and pelvis, and endoscopic examinations of upper and lower gastrointestinal tract were unremarkable. Serum protein electrophoresis was not diagnostic. Antinuclear antibodies (ANA) positivity precipitated a rheumatology referral. Clinical examination revealed a right axillary artery bruit. Ultrasonography of the superficial temporal arteries demonstrated a bilateral halo measuring 0.4 mm on the left and 0.7 mm on the right. Both maxillary arteries demonstrated a halo, 0.9 mm on the right and 1.7 mm on the left (Figure 2). A diagnosis of GCA was established and she was started on prednisolone 50 mg daily.
Figure 2 Transverse image of the maxillary artery showing hypoechoic concentric thickening of the intima-media measuring 1.7 mm. Image taken with L4-12t probe on a GE LOQIQ e ultrasonography machine. B mode frequency 8.0 MHz; colour frequency 4.2 MHz; pulse repetition frequency 3.2.
Discussion
We have described two individuals with a typical history of GCA who were diagnosed using ultrasonography. However, in both cases, we also found abnormalities of the maxillary artery in addition to the superficial temporal artery. Historically, when patients have presented with a typical history and have had a negative temporal artery biopsy, they have continued to be treated as having GCA.8 It is plausible that in these individuals, maxillary artery ultrasonography would have been able to establish the diagnosis of GCA in the other terminal branch of the external carotid artery. There is international consensus on the definitions of abnormalities encountered in the superficial temporal artery.5 It is not unreasonable to use the same definitions when imaging the maxillary artery which, along with the superficial temporal artery, is a co-terminal branch of the external carotid artery.
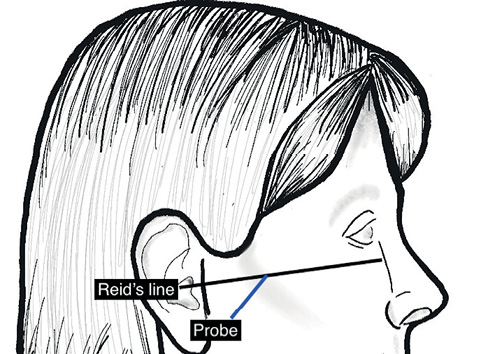
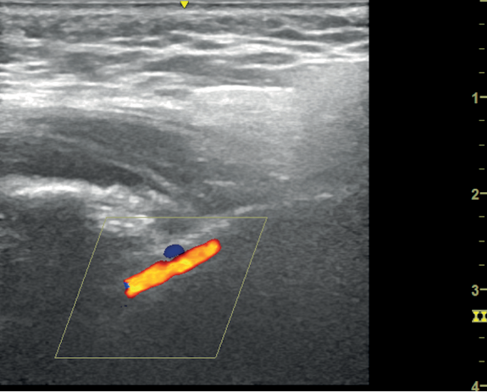
The technique to view the maxillary artery has been described in detail.6 The artery is viewed in the infratemporal fossa between the condylar and coronoid processes of the mandible. The temporomandibular joint must be semi-depressed to allow an optimum window. We achieved this by using the mouthguard used for upper gastrointestinal endoscopy. The longitudinal view of the artery is viewed by keeping the probe at a 45º angle to Reid’s line (a line joining the base of the orbit to the top of the external auditory meatus), which represents the base of the skull (Figure 3). The artery is not always visible because of a normal anatomical variation where it lies deep to the lateral pterygoid muscle. Lepic et al. visualised it in 85% of their patients.6
Figure 3 Schematic showing position of ultrasound probe in the context of Reid’s line (left) to obtain view of maxillary artery (right)
The involvement of the maxillary artery in GCA is supported on clinical and anatomical grounds. Chewing difficulties and jaw claudication are present in up to 45% of individuals with GCA.9 The muscles of mastication are supplied directly by the maxillary artery. The maxillary artery is the larger of the two terminal branches of the external carotid artery, the other one is the superficial temporal artery. Involvement of the maxillary artery may even be an indication of poor outcome. Jaw claudication is thought to be a significant predictor of neuro-ophthalmic manifestations.10 There has been little work on demonstrating involvement of the maxillary artery in GCA, although there is one report where 12% of patients with GCA had maxillary artery involvement on a positron emission tomography scan.3 In both cases described above, the maxillary artery involvement was accompanied by involvement of the superficial temporal artery. There is therefore little doubt that in a typical clinical situation with evidence of superficial temporal arterial inflammation, our findings represent the first demonstration of maxillary artery ‘halo’. It is relevant and important that the size of the halo in both our cases was greater in the maxillary artery than the superficial temporal artery. In a condition where skip lesions lend doubt to negative test results, such changes viewed without superficial temporal involvement could be considered diagnostic of GCA.
Conclusion
The maxillary artery and the superficial temporal artery are co-terminal branches of the external carotid artery. GCA has an affinity for branches of the external carotid artery. Ultrasonography has been used successfully to demonstrate involvement of the superficial temporal artery.11 This is the first demonstration of concentric hypoechoic vessel wall oedema of the maxillary artery using bedside colour doppler ultrasonography. 
Acknowledgements
We thank Dr Sarah Bingham for her appraisal of the manuscript and Emily Ducker for the line drawing.
