Introduction
Since the World Health Organization declared the COVID-19 global pandemic in early March 2020, over 153 million coronavirus cases and 3.2 million deaths were reported worldwide.1 The effective and rapid roll-out of the COVID-19 vaccine in the UK and other parts of the world has brought hope that the pandemic is coming to an end.2,3 Unfortunately, the long-term devastating effects of COVID-19 are here to stay. There has been increasing attention recently about the long-term sequel of COVID-19 called ‘Long COVID’ or post COVID-19 syndrome. Long COVID or post COVID-19 syndrome has been defined as ‘signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis’.4 The epidemiology, clinical characteristics, pathogenesis, and complications of patients with acute COVID-19 have been explicitly described, but the long-term consequences and predictors of the illness remain largely unclear.
A recent study showed that multiple acute COVID-19 symptoms during the first week of illness predicted long COVID.5 Other studies have reported consequences of COVID-19 infection up to 6 months.6,7 Thus, we conducted an observational study with the aim to describe the long COVID symptoms at 3, 6 and 9 months and to determine the risk factors predisposing the development of long COVID following patient hospital discharge.
Methods
We included all patients with confirmed COVID-19 admitted to a district general hospital, in the UK, between 15 February 2020 and 31 July 2020. All patients tested positive on admission for SARS-CoV-2 on a reverse transcriptase polymerase chain reaction assay after a nasopharyngeal swab. Four of the authors contacted all patients via telephone after hospital discharge between 5 January 2021 and 22 January 2021. We carried out a cross-sectional survey of patients using a standardised questionnaire. Subsequently, we assessed patients’ presence and severity of long COVID symptoms at 9 months after their hospital discharge following hospital admissions with acute COVID-19 illness. During the same telephone survey, all patients interviewed were asked to recollect their symptoms for long COVID at 3 and 6 months. Patients who did not answer our initial telephone calls were re-contacted at least three times. Patients who had difficulty hearing, dementia, care home residents, and those unwilling to participate were excluded.
This study was conducted by a group of researchers involved in the direct care of COVID-19 patients during the pandemic. Due to the vulnerable participants involved during the pandemic, interviews were conducted only over the telephone. The retrospective observational study protocol was approved by the Local Review Board of Research and Clinical Effectiveness Department as a service evaluation project.
Survey design
We ensured uniform, reproducible, quality data collected by using a structured questionnaire. The survey was designed to include questions covering patient demographics (n = 89) and long COVID symptoms (n = 17). The survey was vetted for errors in grammar, syntax, and content by four authors. Following this, it was pilot tested among 10 people. Overall, the survey underwent three rounds of correction. We followed the standard guidelines for reporting survey-based studies.8
We enquired about the presence of the long COVID symptoms at 3, 6 and 9 months or over from hospital admission and their severity at the time of the interview. We used the Medical Research Council (MRC) dyspnoea grading for the severity of breathlessness. The rest of the patients’ symptoms severity was graded on a visual analogue scale (VAS) of 1–10, with 10 being the most severe. Chest X-rays (CXRs) of patients at hospital admission were reviewed for the presence of COVID-19 viral pneumonia and their correlation with long COVID symptoms. All the CXRs included in this study were reported by a radiologist.
Researchers discussed the patients’ survey experiences daily during the interview period to prevent any potential discrepancy. The lead researcher coordinated the data collection and interview process to ensure consistency was maintained and high-quality data was obtained.
We used Microsoft Excel to document anonymised data for analysis. Multiple sources, including hospital portals for case notes, blood and radiology investigations, were used to collate information required for the study.
Logistic regression analyses
To evaluate the association of risk factors, logistic regression models were fitted for long COVID symptoms at 3, 6 and 9 months. Additional models were also used for the three main long COVID symptoms: breathlessness, fatigue and cough. Variables such as age, sex, diabetes, intensive care unit (ITU)/high-dependency unit (HDU) admission, length of hospital stay, comorbidity, C-reactive protein (CRP), serum albumin and presence of COVID-19 pneumonia were analysed. Length-of-stay in hospital and CRP at admission were dichotomised to avoid large values being too influential. The set of risk factors considered are summarised in the Supplementary Table 1a.
In each case, the set of univariable models involving each single risk factor in turn and a full multivariable model involving all risk factors simultaneously were fitted. In addition, backward variable selection based on likelihood ratio tests at a 5% significance level was used to identify the most parsimonious model.
Results
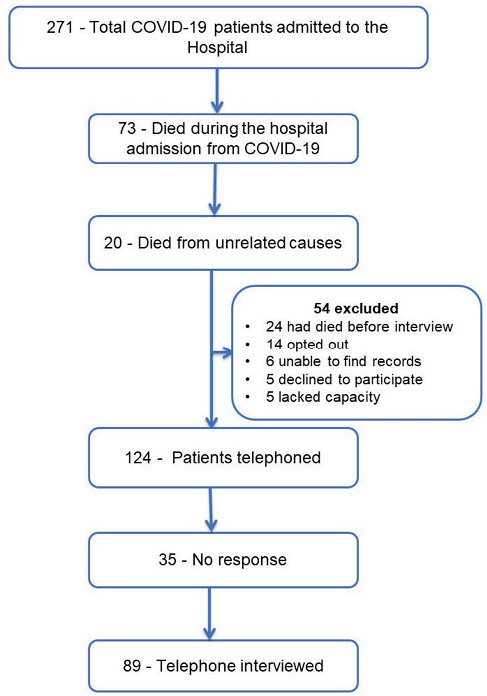
Out of 271 hospital admissions with acute COVID-19, 73 (27%) patients had died during the hospital stay directly related to COVID-19. Twenty patients died of unrelated medical conditions, but COVID-19 was a contributory factor as per their death certificate. Twenty-four patients had died at the time of the interview, 9 months following their hospital admission. However, their cause and time of death are unknown due to lack of access to their death certificates. Thirty patients were excluded (14 opted out as per the NHS ‘opt-out’ policy, five lacked capacity, five declined to participate, records could not be located for six) (Figure 1). Out of the remaining 124 patients, 35 did not answer despite multiple attempts. The remaining 89 patients were interviewed. The response rate was 71%. The mean duration from hospital admission to the interview was 283 days (9.4 months).
Figure 1 Flow chart of patients admitted with COVID-19
The most common comorbidities in our study were hypertension (28, 31%), diabetes (24, 27%) and chronic lung diseases such as asthma and chronic obstructive pulmonary disease (27, 30%) (Table 1).
Table 1 Characteristics of the patients with long COVID after hospital discharge with COVID-19 (n = 89)
|
|
|
|
Mean age, years
|
67.5
|
|
Male, number (%)
|
54 (61%)
|
|
Female, number (%)
|
35 (39%)
|
|
Mean duration of hospital stay, days
|
17
|
|
ITU/HDU admission, number (%)
|
10 (11%)
|
|
|
|
Caucasian
|
85 (96%)
|
|
Asian
|
1 (1%)
|
|
Mixed
|
1 (1%)
|
|
Ethnicity, not known
|
2 (2%)
|
|
|
|
Diabetes
|
24 (27%)
|
|
Hypertension
|
28 (31%)
|
|
Ischemic heart disease
|
12 (13%)
|
|
Congestive cardiac failure
|
5 (0.06%)
|
|
Asthma
|
14 (16%)
|
|
COPD
|
13 (15%)
|
|
Malignancy
|
13 (15%)
|
In total, 55 (62%) patients had long-COVID symptoms over 3 months, out of which 37 were male, and 18 were female. Seventeen male and females each did not have long COVID. Forty-six patients had long COVID symptoms for 6 months. Out of 89 patients interviewed, 75 (84%) patients were admitted to the hospital with acute COVID-19, 9 months before the interview date. Of these, 37 (49%) patients had long COVID symptoms over 9 months from their hospital admission. Out of 37 patients, 30 had multiple long COVID symptoms. Breathlessness, fatigue and cough were the three most common long COVID symptoms at 3 months and they remained predominant symptoms also at 6 and 9 months (Figure 2).
Figure 2 Incidence of long COVID symptoms
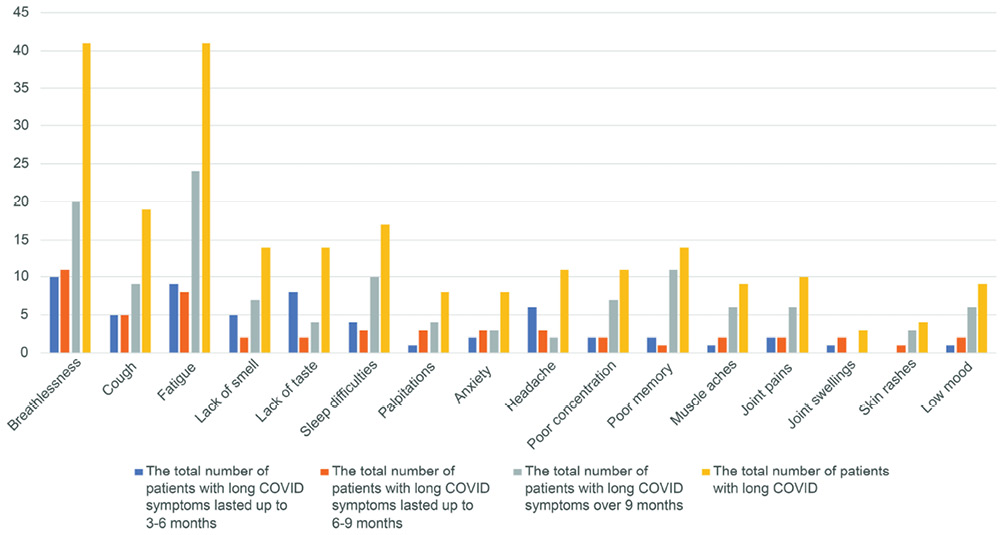
Out of a total of 41 patients with breathlessness as a long COVID symptom, nearly half of the patients (20) continued to have breathlessness at 9 months with a mean severity of 3.2 on the MRC dyspnoea scale. Notably, one patient had breathlessness, fatigue and sleep problems lasting 327 days (10.9 months). Similarly, 58% (24) of patients had fatigue that persisted over 9 months with a mean severity of 5.8 on VAS (1–10) at 9 months or over.
Long COVID predictors at 3, 6 and 9 months
There was no significant difference in mean hospital stay for patients with long COVID symptoms (12.96 days) compared with patients with no long COVID (13.94 days). Out of 55 patients with long COVID symptoms at 3 months, 40 patients (73%) had COVID-19 viral pneumonia on admission CXR, whereas 33 out of 46 patients (72%) who had long COVID symptoms for 6 months had COVID-19 viral pneumonia. Of 37 patients who had long COVID over 9 months, 27 (73%) had viral pneumonia on CXR.
ITU/HDU admission and presence of pneumonia on CXR are individually associated with an elevated risk of long COVID symptoms at 3 months. However, in the multivariable analysis, only the presence of pneumonia was statistically significant, with those with pneumonia having four times the odds of long COVID symptoms (p = 0.01). Using backward variable elimination, the model containing only the presence of pneumonia is the most parsimonious (Table 2).
Table 2 Estimated odds ratios (OR) and corresponding 95% confidence intervals (CIs) and p-values for univariable and multivariable models for presence of long COVID symptoms at 3 months
|
|
|
|
|
|
|
|
|
|
Age (years)
|
0.99 (0.96–1.02)
|
0.398
|
0.97 (0.93–1.01)
|
0.129
|
|
Sex (male)
|
2.06 (0.86–5.00)
|
0.106
|
1.02 (0.34–2.91)
|
0.972
|
|
Diabetes
|
1.73 (0.65–4.99)
|
0.280
|
1.35 (0.43–4.53)
|
0.611
|
|
ITU/HDU admission
|
6.46 (1.13–122)
|
0.034
|
3.62 (0.53–73.3)
|
0.207
|
|
Length of stay >14 days
|
1.07 (0.43–2.79)
|
0.881
|
1.47 (0.48–4.71)
|
0.498
|
|
≥3 comorbidities
|
0.97 (0.40–2.32)
|
0.952
|
2.10 (0.60–7.94)
|
0.247
|
|
CRP at admission >75
|
2.13 (0.89–5.17)
|
0.088
|
1.21 (0.37–3.83)
|
0.752
|
|
Albumin at admission
|
0.95 (0.85–1.05)
|
0.302
|
1.00 (0.87–1.15)
|
0.974
|
|
Presence of pneumonia on CXR
|
4.89 (1.99–12.6)
|
<0.001
|
4.05 (1.39–12.7)
|
0.010
|
Using the presence of symptoms at 6 months as the outcome variable gives qualitatively similar results to the 3-month endpoint. However, the effects of ITU/HDU admission [odd ratio (OR) 2.39, 95% confidence interval (CI) 0.62–11.7, p = 0.212] and pneumonia (OR 3.21, 95% CI 1.35–7.91, p = 0.008) were less pronounced. In the multivariable model, no variables were individually significant, and only CXR evidence of pneumonia was retained after backwards elimination.
The association with the presence of symptoms at 9 months was further evaluated. There is a non-significant association with ITU/HDU admission (OR 2.39, 95% CI 0.58–12.1, p = 0.231), and a similar association with the presence of pneumonia (OR 3.30, 95% CI 1.29–8.87, p = 0.012). However, diabetes was also associated with an increased risk of long COVID at 9 months (OR 3.07, 95% CI 1.06–9.81, p = 0.039). In the multivariable model none of the variables was statistically significant. At 6 months, only the model involving CXR evidence of pneumonia was retained after backwards elimination. Estimates for the 6-month and 9-month endpoints are provided in Supplementary Tables 1b and 1c.
ITU/HDU admission (OR 5.03, 95% CI 1.17–34.8, p = 0.029), high CRP at admission (OR 2.52, 95% CI 1.07–6.14, p = 0.035) and CXR evidence of viral pneumonia (OR 3.78, 95% CI 1.57–9.58) were individually associated with an increased risk of breathlessness at 3 months. CXR evidence of viral pneumonia remained statistically significant after adjusting for the other risk factors (OR 3.18, 95% CI 1.12–9.64, p = 0.029) and was the only significant variable (previously important) after model selection.
Length of stay greater than 14 days (OR 3.06, 95% CI 1.09–8.74, p = 0.035) and lower albumin at admission (OR 0.85, 95% CI 0.74–0.96, p = 0.008) were individually associated with increased risk of cough at 3 months. None of the risk factors was statistically significant in the multivariable model for cough. After model selection, length of stay (OR 4.04, 95% CI 1.27–13.7) and evidence of pneumonia in CXR (OR 5.75, 95% CI 1.57–29.0) were the only important variables.
Evidence of pneumonia in CXR (OR 3.22, 95% CI 1.34–8.12, p = 0.008) and ITU/HDU admission (OR 5.58, 95% CI 1.30–38.6, p = 0.020) were associated with a higher chance of fatigue at 3 months based on the univariable analyses. However, in the multivariable model, no risk factor was individually significant. Evidence of pneumonia in CXR was the variable retained through backwards elimination.
Discussion
Although long COVID data are scarce, they are evolving. Research evaluating risk factors of long COVID are limited, and study duration has been restricted to only up to 6 months.6,7 We showed that 55 (62%), 46 (52%) and 37 (49%) patients had long COVID symptoms at 3, 6 and 9 months, respectively, following their hospital admissions. COVID-19 pneumonia was the strongest predictor of long COVID at 3 months and may predict long COVID symptoms for 6 and 9 months. The average number of long COVID symptoms per patient was 4.4, with the maximum number being 11 out of 17 symptoms studied.
We demonstrated that 55 (62%) patients had at least one long COVID symptom at 3 months, which is lower than the study by Carfi et al. (125 patients, 87%).7 This may be explained by a longer follow up of 3 months versus 2 months in the aforementioned, suggesting symptoms wane over time in a subset of individuals. Similarly, fatigue and breathlessness were the two most common long COVID symptoms in our study at 3, 6 and 9 months as in most other studies on the subject. At 6 months, another single-centre study from China found at least one long COVID symptom in 76% of their patients.6 Since this addresses a different ethnic group, it seems plausible that sociodemographic factors may be additional determinants of long-term outcomes, more so later after hospitalisation when factors such as pneumonia have a seemingly declining effect. Similar to our study, the COMEBAC Study Group has also found that just over half of their patients had at least one long COVID symptom at 4 months after their hospital discharge.9 In a Veterans’ Affairs study, burden of respiratory symptoms was most common (28.51 per 1,000 COVID-19 patients), followed by sleep disorders (14.53 per 1,000 COVID-19 patients) at 6 months following hospitalisation.10 Contrarily, sleep disturbances were not that common in our study, though that could be attributed to a different study design.
Long-term sequelae of COVID-19 are not exclusive to SARS-CoV-2. Chronic fatigue had been a frequent long-term symptom in severe acute respiratory syndrome (SARS) in one study, chronic fatigue was a predominant symptom in 40% of the participants at 4 years follow up.11 Similar to our study, depression (33%) and anxiety (30%) were common features of patients with SARS/MERS (Middle East Respiratory Syndrome) after hospital discharge beyond 6 months.12 Despite inherent differences between the various coronaviruses, we may infer that long-term sequela of COVID-19 follow a similar course to SARS and MERS.
Seventy-three per cent of the patients with long COVID symptoms at 9 months had CXR evidence of viral pneumonia at admission, and the majority of them had multi-lobar involvement. The presence of pneumonia at baseline is associated with long COVID symptoms at 9 months, suggesting that these features may potentially be contributed by residual lung changes, which reportedly occurred in 43% and 30% at 3 and 6 months, respectively.13–15 It may also be reflective of general deconditioning and POTS (postural orthostatic tachycardia syndrome), leading to chronic fatigue syndrome.16 Unfortunately, follow-up CXRs were not available to evaluate if our patients with long COVID symptoms at 9 months had persistent lung changes.
We studied multiple variables such as age, sex, diabetes, ITU/HDU admission, length of stay more than 14 days, comorbidities, CRP and albumin at admission, and presence of COVID-19 pneumonia and their association with long COVID. CXR evidence of COVID-19 pneumonia was associated with an increased risk of long COVID at 3 months and was the most important predictor of long COVID symptoms at 6 months and 9 months. ITU/HDU admission and diabetes were individually associated with an elevated risk of long COVID symptoms at 3 and 9 months, respectively. However, these associations were not significant after adjusting for evidence of COVID-19 pneumonia. At 6 months, 72% of patients with long COVID symptoms had the presence of pneumonia, which is similar to the proportion at 3 months (73%). Although we have not assessed whether patients’ presenting clinical features for acute COVID-19 correlate with long COVID, none of the variables in our study, such as diabetes, low albumin at admission or multiple comorbidities, were associated with long COVID. Similar findings have been reported in a Mediterranean cohort study that assessed post-COVID symptoms at 2.5 months after the onset of acute illness.17 However, this does not discount the possibility that baseline patient-specific factors, such as sociodemographic determinants, pain sensitisation and other poorly understood variables, contribute to a long-hauler status.
Notably, a large prospective self-reported app-based study in 4,182 COVID-19 positive individuals found that multiple COVID-19 presenting symptoms in the first week (fatigue, breathlessness, headache, hoarse voice and myalgia) were strongly associated with long COVID at 28 days [OR 3.53 (2.76–4.50)].18 However, loss of smell was the only symptom associated with long COVID in patients aged more than 70 years. Older age (more than 70 years), women and asthma predicted long COVID at 28 days. Older patients were under-represented in this study, whereas patients in our study were older (mean age 67 years) and were hospitalised with acute COVID-19.
Long COVID symptoms in our study, fatigue, myalgia, arthralgia, sleep difficulties, and sicca symptoms are common in several rheumatological conditions such as chronic fatigue syndrome, fibromyalgia and systemic auto-immune rheumatic diseases. Hence long COVID will remain a diagnostic challenge, given the similarities it shares with many common rheumatic diseases.
To our knowledge, this study is the longest follow-up study for 9 months, assessing long COVID symptoms and their risk factors so far. Our study has some limitations inherent to a self-reported questionnaire and a recall bias. Patients had to recall their symptoms several months after their hospital discharge. This is a single-centre study with a relatively small number of patients. Our study was largely representative of a Caucasian population, and it seems plausible that long COVID outcome differs in other ethnic groups – an area that merits further exploration. Moreover, with a long duration between patients admitted with acute COVID-19 infection to the time of the interview, patients could have had other contributing factors for long COVID.
The relatively small sample size means limited power to detect risk factors with mild to moderate or strong effects associated with rarer risk factors. As such, a non-significant association does not imply the absence of an effect. Conversely, no adjustment for multiplicity of testing has been applied to the presented p-values meaning the nominal statistical significance should be viewed with caution. However, we hope that our study will pave the way for further research, particularly evaluating the predictors of long COVID in large patient cohorts. This will help to identify patients at risk and provide holistic care and support for long COVID in time. Moreover, our research data will guide healthcare providers to set up appropriate services to address the current problem based on demand locally and in the wider community.
In conclusion, this study reinforces that the adverse effects of long COVID symptoms continue to hamper patients’ recovery at 9 months and beyond. Although fatigue and breathlessness are the two most common symptoms in our study, long COVID could present with multiple other symptoms as described, which could pose a diagnostic and management challenge as they mimic some of the common rheumatological conditions. Viral pneumonia at admission may predict long COVID symptoms in Caucasians at 3, 6 and 9 months. Large, prospective research studies are required to confirm these findings. 
Acknowledgements
Dr Tak Kit Poon and Dr Akshita Dandawate contributed to data collection.
Online Supplementary Material
Supplmentary data are available with the online version of this paper, which can be accessed at https://www.rcpe.ac.uk/journal.
References
1 World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. WHO. https://covid19.who.int/ (accessed 30/04/21).
2 GOV.UK. Coronavirus (COVID-19). https://www.gov.uk/coronavirus (accessed 30/04/21).
3 Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/index.html (accessed 30/04/21).
4 National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19: NICE guideline [NG188]. 2020. www.nice.org.uk/guidance/ng188 (accessed 30/04/21).
5 Sudre CH, Murray B, Varsavsky T et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626–31.
6 Huang C, Huang L, Wang Y et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–32.
7 Carfì A, Bernabei R, Landi F. Gemilli against COVID-19 post-acute care study group. persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–5.
8 Gaur PS, Zimba O, Agarwal V et al. Reporting survey based studies – a primer for authors. J Korean Med Sci 2020; 35: e398.
9 Morin L, Savale L, Pham T et al. Writing Committee for the COMEBAC Study Group. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325: 1525–34.
10 Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequalae of COVID-19. Nature 2021; 594: 259–64.
11 Lam MH, Wing YK, Yu MW et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 2009; 169: 2142–7.
12 Ahmed H, Patel K, Greenwood DC et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med 2020; 52: jrm00063.
13 Hui DS, Joynt GM, Wong KT et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005; 60: 401–9.
14 Parry AH, Wani AH, Shah NN et al. Medium-term chest computed tomography (CT) follow-up of COVID-19 pneumonia patients after recovery to assess the rate of resolution and determine the potential predictors of persistent lung changes. Egypt J Radiol Nucl Med 2021; 52: 55.
15 Han X, Fan Y, Alwalid O et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021; 299: E177–86.
16 Johansson M, Ståhlberg M, Runold M et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep 2021; 3: 573–580.
17 Moreno-Pérez O, Merino E, Leon-Ramirez JM et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect 2021; 82: 378–83.
18 Sudre CH, Murray B, Varsavsky T et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626–31.
