Introduction
The COVID-19 pandemic has caused nearly 46 million cases globally with nearly 1.2 million deaths.1 It has been hypothesised that the principal mode of transmission of the causative SARS-CoV-2 virus involves exposure to respiratory droplets during close contacts with infected individuals.2 However, SARS-CoV-2 is also thought to have airborne or aerosolised transmission under special circumstances, such as being in enclosed spaces, spaces having inadequate ventilation including the use of centralised air conditioning, and, of particular importance in healthcare facilities, aerosol-generating procedures.2,3 This characteristic of the virus coupled with a significant proportion of asymptomatic carriers may contribute to its capability of transmission.2
Hospitals and other healthcare facilities tend to have a combination of these factors present that can facilitate transmission of COVID-19 despite adequate infection control practices. There have been multiple reports of nosocomial transmission of COVID-19 even though the rates remain quite low.4,5 The use of wards with multiple patients sharing a common air space, common air conditioning, common washrooms that may generate aerosols, as well as the potential of contact between hospital staff with patients during routine clinical care potentially increases the risk of nosocomial COVID-19. We report a cohort of 15 patients with possible nosocomial COVID-19 over a period of 48 days from a tertiary care centre in South India. We reviewed all the patients who were diagnosed to have COVID-19 3 days after admission for a different reason. We then performed a root cause analysis to assess the effectiveness of the infection control program at the hospital.
Methods
Study design and setting
This study was conducted as a retrospective cohort study involving all patients suspected to have nosocomial COVID-19 in a 500 bedded tertiary care hospital in South India during a 48-day period between 23 August 2020 to 9 October 2020. We defined nosocomial COVID-19 as any patient having symptoms, signs or radiological chest imaging suggestive of COVID-19 with a positive SARS-CoV-2 reverse-transcription polymerase chain reaction (RT-PCR) at least 72 hours (3 days) after hospital admission, based on a previous study.4 Our study was exempted from full review by the Institutional ethical board (vide letter 01-15/07/2021) due to minimal risk to patients and as the study involved only information collected and analysed as part of routine hospital infection control practice. This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.6
Patient characteristics and data collection
We identified all patients with COVID-19 in whom the first positive RT-PCR test result was obtained at least 72 hours (3 days) after hospital admission. Post discharge patients were not followed up for possibility of nosocomial COVID-19. The medical records of the patients, their clinical features, and movement within the hospital were studied by a single physician (BR) to determine whether the infection was most likely acquired prior to hospitalisation or after hospital admission, based on the timing of symptoms, RT-PCR, and potential exposures within or outside the hospital based on a previous similar study (Table 1).4
Table 1 Criteria for classifying patients as community acquired or nosocomial COVID-194
|
|
|
|
Definitely community acquired
|
- Symptoms present during admission and first RT-PCR test positive on days 3–7 after admission
|
|
Likely community acquired
|
- Symptoms present during admission, first RT-PCR test positive on days 8–14 after admission, and not tested prior to day 8
- Symptom onset and first RT-PCR test positive on days 3–7 after admission, with no known exposure on day 1 or 2
- Symptom onset and first RT-PCR test positive on days 8–14 after admission, with known exposure in the community prior to hospitalisation (in the 14 days preceding symptom onset)
|
|
Likely hospital acquired
|
- Symptom onset and first RT-PCR test positive on days 3–7 after admission, with known exposure in the hospital on day 1 or 2, and no known exposure in the community prior to hospitalisation
- Symptom onset and first RT-PCR test positive on days 8–14 after admission, with no known exposure in the community prior to hospitalisation (in the 14 days preceding symptom onset)
|
|
Definitely hospital acquired
|
- Symptom onset and first RT-PCR test positive on or after 15th day of hospital admission
|
|
Unknown
|
- None of the above criteria
|
The potential COVID-19 exposure for the patients was studied by cross referencing the overlap of locations and periods of infectivity between the patient, with either hospital staff who worked in the same ward and tested positive for COVID-19 or with other patients admitted in the same ward who tested positive for SARS-CoV-2. A definite exposure was taken as the sharing of the same air space during the infectivity window of a patient or staff member who tested positive for COVID-19. For example, if a staff member working on a particular ward tested positive for COVID-19, and a patient from that ward later tested positive for COVID-19, an analysis was performed to assess whether there was sharing of air space between this patient and the staff member during the staff member’s infectivity window. The infectivity window was taken as 72 hours prior to symptom onset or a positive RT-PCR test as per governmental guidelines.7 The duration of exposure, nature of exposure whether direct contact or not, and physical distance between the infected person and the contact were not studied due to unavailability of data given the retrospective nature of the study. The patients or staff who tested positive for COVID-19 were immediately isolated and hence no exposure was deemed possible after test positivity.
Infection control practices
The routine Infection Control and Testing Standards during the study period remained the same as per governmental guidelines, and included screening of all patients for COVID-19 symptoms on admission, the use of RT-PCR initially for all symptomatic patients and SD Biosensor Rapid antigen test for all asymptomatic patients who were suspected to have COVID-19 based on exposure or risk factors and this was carried out as per governmental guidelines.8 There were 100 beds that were dedicated for COVID-19 patients in admission units with airborne infection isolation rooms, personal protective equipment (PPE) including universal masking of both patients and hospital staff, with additional face shields for all staff in accordance with the World Health Organization and governmental guidelines, along with restriction of visitors.
The governmental protocol to discontinue isolation precautions for COVID-19 patients required a negative SD Biosensor COVID-19 rapid antigen test result from nasopharyngeal swab samples that was obtained on the tenth day after symptom onset if they are asymptomatic on the day of testing or if symptoms persist, one day after the resolution of symptoms.9 If despite a negative RT-PCR, the patient had a productive cough or required mechanical ventilation, they were pre-emptively placed in isolation with all precautions.
Statistical analysis
Descriptive analyses of patient and hospitalisation characteristics were performed using Microsoft Excel 2016.
Results
During the study period, 173 inpatients were diagnosed to have COVID-19. Out of these, 15 (8.6%) patients who fulfilled the criteria for likely or definitely hospital-acquired COVID-19 infection were identified. There were 12 male and 3 female patients. These patients were designated with numbers 1 to 15. These 15 patients were segregated based on their locations from the time of admission to the time of diagnosis of COVID-19 into 6 wards in the hospital designated by numbers 1 to 6 (Figure 1).
In the identified 15 patients, the mean hospital stay was 15.6 ± 8.9 days. Seven patients had a definite hospital-acquired COVID-19 as per the pre-determined criteria, while 8 patients had a likely hospital acquired infection. A probable exposure to a hospital staff who was in their infective window was identified in 12 patients while in 3 patients (patient numbers 1, 2 and 12), a potential source of infection could not be identified. In these 12 patients, the mean incubation period, calculated with respect to the earliest exposure to staff members in the infectivity period was 8.8 ± 4 days (Table 2). A total of 11 (73%) patients were located in ward 1. One patient each were located in wards 2, 3, 5 and 6. One patient (patient number 9) had an exposure in both wards 1 and 4. In patient number 14, the exposure likely occurred at the time of admission as the admitting resident physician was in their infective window (Figure 1).
Table 2 Epidemiological details of the potentially infective staff members
|
|
|
|
|
|
|
|
Ward 1
|
A
|
Nurse
|
19-09-2020
|
21-09-2020
|
10
|
|
B
|
Nurse
|
16-09-2020
|
18-09-2020
|
|
C
|
Nurse
|
24-09-2020
|
26-09-2020
|
|
D
|
Nurse
|
19-09-2020
|
21-09-2020
|
|
E
|
Housekeeping
|
17-09-2020
|
19-09-2020
|
|
F
|
Housekeeping
|
16-09-2020
|
18-09-2020
|
|
Ward 2
|
G
|
Nurse
|
31-08-2020
|
02-09-2020
|
3
|
|
H
|
Nurse
|
31-08-2020
|
02-09-2020
|
|
I
|
Nurse
|
31-08-2020
|
02-09-2020
|
|
J
|
Nurse
|
31-08-2020
|
02-09-2020
|
|
K
|
Nurse
|
31-08-2020
|
02-09-2020
|
|
L
|
Nurse
|
09-09-2020
|
11-09-2020
|
|
M
|
Non-medical
|
31-08-2020
|
02-09-2020
|
|
Ward 3
|
N
|
Nurse
|
23-09-2020
|
25-09-2020
|
4
|
|
O
|
Nurse
|
22-09-2020
|
24-09-2020
|
|
Ward 4
|
P
|
Housekeeping
|
15-09-2020
|
17-09-2020
|
3
|
|
Ward 5
|
Q
|
Nurse
|
22-09-2020
|
24-09-2020
|
3
|
|
Independent of ward
|
R
|
Doctor
|
03-10-2020
|
05-10-2020
|
3
|
Figure 1 Graphical representation showing the distribution of the patients, their respective locations, dates of potential exposure, date of test positivity and the infectivity windows of the staff
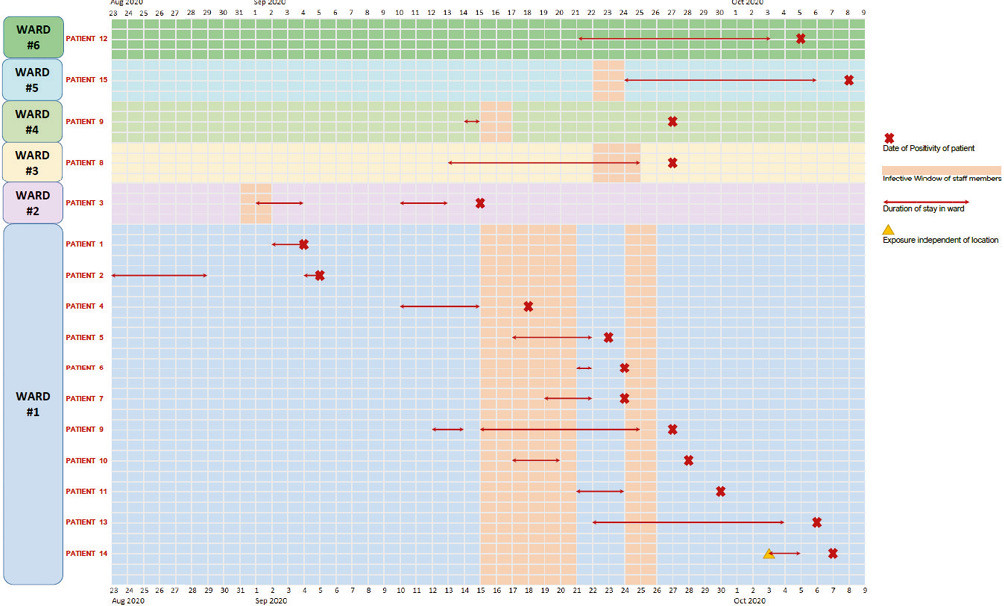
During this same study period, 121 hospital staff members were diagnosed with COVID-19. Out of these, 18 (14.9%) hospital staff members were identified who could have been the potential source of infection for these 15 patients based on the overlap of location of the staff and the patients, and their infectivity windows. These consisted of 13 staff nurses, 3 housekeeping staff, 1 non-medical staff and 1 doctor. Out of these 18 staff members, 17 members together produced an infectivity window of 14 days in 6 wards, where the 15 patients were located (Figure 1). One staff member was a doctor who produced an infective window of 3 days independent of the location as he was not assigned to any particular ward. In ward 1, there were 6 staff members identified who together created an infective window of 10 days. In ward 2, there were 7 staff members identified who together created an infective window of 3 days. In ward 3, there were 2 staff members identified who together created an infective window of 4 days. In ward 4, there was 1 staff member identified who created an infective window of 3 days. In ward 5, there was 1 staff member identified who created an infective window of 3 days and in ward 6, there were no potentially infective staff identified (Table 2).
The patients with nosocomial COVID-19, their location and duration in the respective wards, the date of positivity and incubation periods of these patients, and the infectivity window of staff in those respective locations are summarised in Figure 1. The patient details are summarised in Table 3 and the staff details are summarised in Table 2.
Table 3 Epidemiological details of the patients
|
|
|
|
|
|
|
|
|
|
1
|
Female
|
Ward 1
|
28-08-2020
|
NK
|
04-09-2020
|
8
|
NK
|
|
2
|
Female
|
Ward 1
|
16-08-2020
|
NK
|
05-09-2020
|
21
|
NK
|
|
3
|
Male
|
Ward 2
|
14-08-2020
|
01-09-2020
|
15-09-2020
|
33
|
14
|
|
4
|
Female
|
Ward 1
|
10-09-2020
|
15-09-2020
|
18-09-2020
|
9
|
4
|
|
5
|
Male
|
Ward 1
|
12-09-2020
|
17-09-2020
|
23-09-2020
|
12
|
7
|
|
6
|
Male
|
Ward 1
|
21-09-2020
|
21-09-2020
|
24-09-2020
|
4
|
4
|
|
7
|
Male
|
Ward 1
|
18-09-2020
|
19-09-2020
|
24-09-2020
|
7
|
6
|
|
8
|
Male
|
Ward 3
|
08-09-2020
|
22-09-2020
|
27-09-2020
|
21
|
6
|
|
9
|
Male
|
Ward 1
|
12-09-2020
|
15-09-2020
|
27-09-2020
|
16
|
13
|
|
10
|
Male
|
Ward 1
|
17-09-2020
|
17-09-2020
|
28-09-2020
|
12
|
12
|
|
11
|
Male
|
Ward 1
|
21-09-2020
|
21-09-2020
|
30-09-2020
|
10
|
10
|
|
12
|
Male
|
Ward 6
|
17-09-2020
|
NK
|
05-10-2020
|
19
|
NK
|
|
13
|
Male
|
Ward 1
|
11-09-2020
|
24-09-2020
|
06-10-2020
|
26
|
13
|
|
14*
|
Male
|
Ward 1
|
03-10-2020
|
03-10-2020
|
07-10-2020
|
5
|
3
|
|
15
|
Male
|
Ward 5
|
08-09-2020
|
24-09-2020
|
08-10-2020
|
31
|
14
|
Discussion
This study was conducted as a retrospective cohort study involving patients suspected to have likely or definite hospital-acquired COVID-19 in a tertiary care hospital in Kerala, India. We found that 15 patients had likely or definite nosocomial COVID-19. These 15 patients represented only 8.6 % of the total number of COVID-19 cases admitted in the hospital during the study period.
Our study found an incidence of 8.6% of nosocomial COVID-19 infections compared with the total burden of COVID-19 in our hospital during the same time period. This was slightly higher than the findings reported by Rhee et al.4 who found an incidence of less than 1%. Our findings are substantially lower than the results of a review by Zhou et al.10 which suggested that up to 44% of COVID-19 infections may be hospital acquired. However, it may be relevant that this review was limited to studies that were conducted early on in the COVID-19 outbreak in Wuhan, China, before the recognition of the SARS-CoV-2 virus and implementation of adequate infection control.
Hospital staff are at an increased risk of being exposed to the causative SARS-CoV-2 and this factor could also potentially have a role in nosocomial transmission of COVID-19. During the Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome (SARS) coronavirus outbreak, nosocomial outbreaks of both these infections were thought to have played a major role in the amplification and spread of the disease.11
Similarly, infection control with respect to COVID-19 is also a difficult task due to the nature of its transmission. Jones et al.12 studied the relative contributions of transmission routes in COVID-19 among healthcare workers directly providing care for patients and found that the predominant route was through inhalation (57%), followed by droplets (35%) and a small proportion by direct contact (8.2%). In our hospital, as per governmental guidelines, the use of a combination of N95 masks and face shields had been made mandatory since early on in the COVID-19 pandemic. The identification of hospital-acquired COVID-19 cases despite the use of universal masking and face shields suggests the possibility of direct contact while administering patient care and fomite transmission being one of the predominant drivers of transmission in our study. As a result of our findings, we have implemented a policy of universal gloving for all staff who are involved in the direct care of patients. This policy involves the use of disposable gloves during contact with any inpatient that are discarded immediately after use. The use of gloves when dealing with potential COVID-19 patients was already in the institutional PPE guidelines, which was expanded to all patients in the hospital due to the ongoing pandemic and potential for fomite transmission. Chang et al. reported in a systematic review and meta-analysis of 8 studies that demonstrated that implemented universal gloving alone showed a significant association with decreased incidence of hospital-acquired infections (IRR 0.77; 95% CI 0.67–0.89).13
Our study also identified a large cluster of 12 patients in a single location, which turned out to be the general medical ward, where there is maximum concentration of patients in the hospital with an open cubicle model, and sharing of a common centralised air system. This could have played a role in the transmission given the predominantly inhalation mode of transmission reported by Jones et al.13 Wong et al.14 reported early in the pandemic from Hong Kong that there was no transmission in a general ward in their hospital after extensive contact tracing and follow up of all the patients and hospital staff who had come into contact with an index case of COVID-19. However, that study concentrated on the transmission from a COVID-19 patient to other patients and to the staff members rather than from the staff member to the patients. They also had only a single positive case of COVID-19 and the patient stayed in the studied ward for a period of only approximately 35 hours. Our study on the other hand consisted of multiple windows of infectivity in the general ward, with both staff and patients being infective during these windows, which could have led to the cluster. As a result of our findings that suggested a breach in the contact precautions by the staff members, we have planned to strengthen the infection control practices in the wards identified to have been potential areas of transmission, with the appointment of a full-time dedicated infection control nurse in those areas.
Our study does have its limitations. First, our sample size is relatively low and as the number of total cases increase, the proportion of nosocomial COVID-19 may also increase. Second, the possibility of asymptomatic bystanders being the drivers of transmission could not be studied. Third, all cases among admitted patients and hospital staff members may not have been identified as only symptomatic patients underwent testing. Due to the relatively high proportion of asymptomatic cases of COVID-19, cases could have been missed in our study. Fourth, we did not study the patients after discharge. There could have been patients who developed symptoms after discharge, but we were unable to look at these patients. However, we did not admit any COVID-19 patients who had been discharged from our own hospital in the preceding 14 days. Finally, patients transferred in from other centres were not included in the nosocomial category, but were considered as community-acquired due to the retrospective nature of the study and unavailability of data concerning the risk factors in the previous hospital.
In conclusion, despite the admission of a large number of COVID-19 patients and a relatively large proportion of hospital staff members who tested positive for the disease, the proportion of nosocomial COVID-19 in our centre remained low. The predominant route of this transmission could have been through direct contact or fomite transmission from infected staff to these patients. The newly instituted policy of universal gloving coupled with the already existing practice of universal N95 masking and face shield use, could potentially bring down the rate of nosocomial COVID-19 even further. 
Acknowledgements
We would like to thank the hospital management in assisting with the contact tracing and facilitation of this study.
References
1 COVID-19 weekly epidemiological update. World Health Organization. https://www.who.int/docs/default-source/coronaviruse/situation-reports/w... (accessed 05/11/21).
2 Scientific Brief: SARS-CoV-2 and potential airborne transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov.... (accessed 05/11/21).
3 Lu J, Gu J, Li K, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis 2020; 26: 1628– 31.
4 Rhee C, Baker M, Vaidya V et al. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA 2020; 3: e2020498.
5 Taylor J, Rangaiah J, Narasimhan S et al. Nosocomial COVID-19: experience from a large acute NHS Trust in South-West London. J Hosp Infect 2020; 106: 621– 5.
6 Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; 13: S31– 4.
7 COVID-19 contact tracing and quarantine guidelines. Health and Family Welfare department, Govt of Kerala. https://dhs.kerala.gov.in/wp-content/uploads/2020/08/1598163078157_Guide... (accessed 05/11/21).
8 COVID-19-revised testing guidelines. Health and Family Welfare department, Govt of Kerala. https://dhs.kerala.gov.in/wp-content/uploads/2020/08/1597486460508_COVID... (accessed 05/11/21).
9 Revised discharge guidelines for COVID-19 patients. Health and Family Welfare department, Govt of Kerala. https://dhs.kerala.gov.in/wp-content/uploads/2020/10/Gudelines-Revised-D... (accessed 05/11/21).
10 Zhou Q, Gao Y, Wang X et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med 2020; 8: 629.
11 Sikkema RS, Pas SD, Nieuwenhuijse DF et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis 2020; 20: 1273– 80.
12 Jones RM. Relative contributions of transmission routes for COVID-19 among healthcare personnel providing patient care. J Occup Environ Hyg 2020; 17: 408– 15.
13 Chang NN, Kates AE, Ward MA et al. Association between universal gloving and healthcare-associated infections: a systematic literature review and meta-analysis. Infect Control Hosp Epidemiol 2019; 40: 755– 60.
14 Wong SCY, Kwong RT, Wu TC, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect 2020; 105: 119–27.
