A 72-year-old female presented with right arm weakness and motor aphasia to our outpatient transient ischemic attack clinic. The only known vascular factor was a background of hypercholesterolaemia. She had no other cardiovascular risk factors. Clinical examination was unremarkable. Manual pulse and electrocardiogram (ECG) confirmed the patient was in sinus rhythm, no carotid bruit was identified. There were no abnormal heart sounds. MRI head was undertaken that confirmed left basal ganglia infarct. There was no carotid artery stenosis on duplex ultrasonography. Paroxysmal atrial fibrillation was excluded on 72-hour ambulatory ECG monitoring. Transoesophageal echocardiogram (ECHO) was performed, which confirmed rheumatic mitral valve with only mild stenosis and regurgitation and no evidence of left atrial or appendicular clot or thrombus. Transoesophageal ECHO also identified small patent foramen ovale/stretched atrial septal defect, but the patient had no history of pulmonary embolism or deep venous thrombosis. Thickened mitral leaflets with multiple filiform fronds were noted at the tips of mitral valves. Aortic valve leaflets were also mildly thickened with good excursion. Multiple Lambl’s excrescences were noted on aortic valves, some were calcified. There was no evidence of atherosclerosis of the thoracic aorta. Antinuclear antibody and antineutrophil cytoplasmic antibody tests were negative, erythrocyte sedimentation rate was not raised and syphilis serology was negative. Coagulopathy results (lupus anticoagulant, anticardiolipin antibodies) came back within normal limits. The cardio-stroke multidisciplinary team suggested anticoagulation, which was initiated. Following investigations and once all the results were collated a diagnosis of embolic stroke was given, secondary to Lambl’s excrescence. Follow up was arranged at 6 weeks, which the patient attended. The patient did not report any further events, no further cerebrovascular ischemic events were identified in the follow up.
Figure 1 MRI head showing hyperintensity in the left lentiform nucleus (arrow)
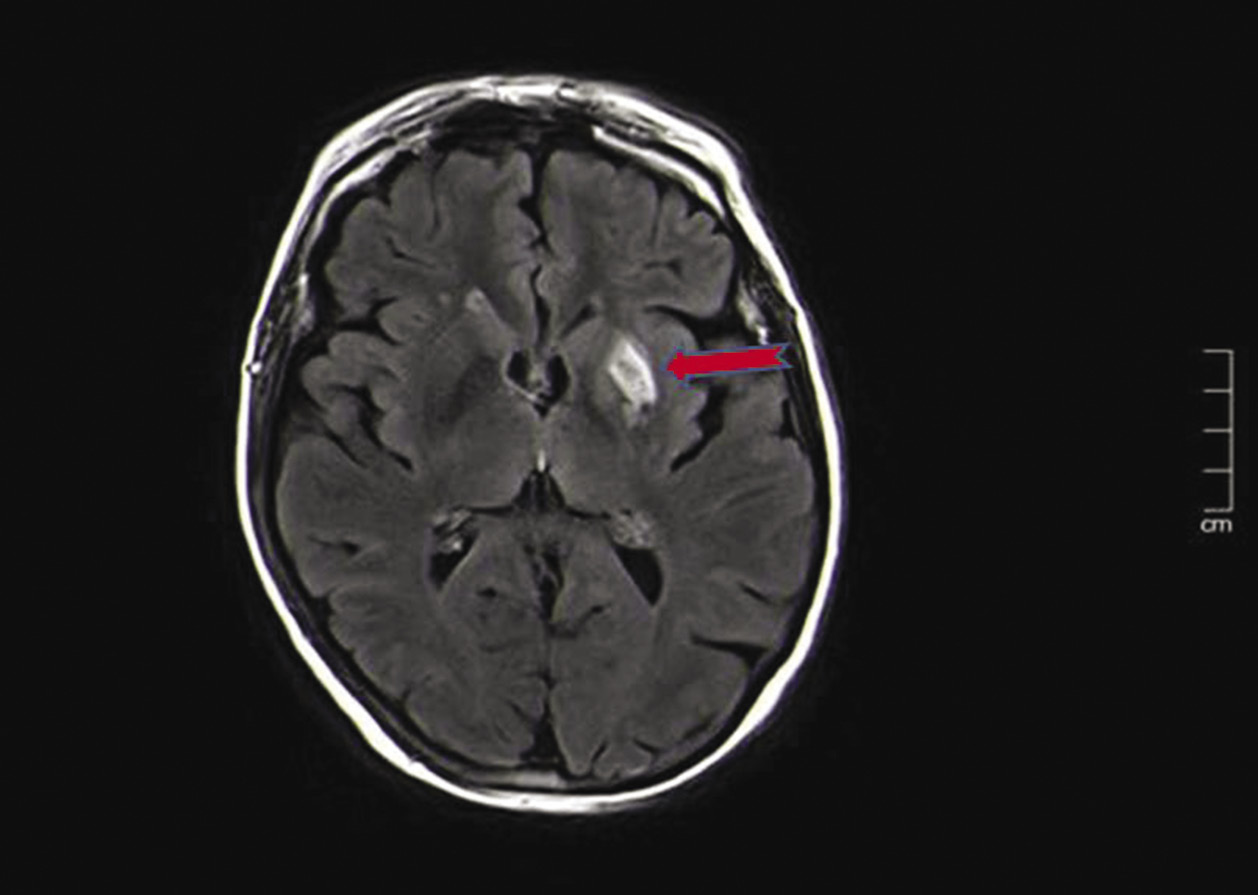
Figure 2 Echocardiogram demonstrating Lambl’s excrescence on aortic valves (arrow)
References
1 Lambl VD. Papillare excrescenzen an der semilunar-klappe der aorta [in German]. Wien Med Wochenschr 1856; 6: 244–7.
2 Wu TY, Gerber IL, Roxburgh RH. Thrombo-embolic cerebral infarction secondary to giant Lambl’s excrescence. J Clin Neurosci 2013; 20: 1632–4.
3 Aziz F, Baciewicz Jr FA. Lambl’s excrescences: review and recommendations. Tex Heart Inst J 2007; 34: 366–8.
4 Jaffe W, Figueredo VM. An example of Lambl’s excrescences by transesophageal echocardiogram: a commonly misinterpreted lesion. Echocardiography 2007; 24: 1086–9.
5 Davogustto G, Fernando RR, Loghin C. Lambl’s excrescence, migrainous headaches, and “tiger stripes”: puzzling findings in one patient. Tex Heart Inst J 2015; 42: 70–2.
6 Nighoghossian N, Trouillas P, Perinetti M et al. Lambl’s excrescence: an uncommon cause of cerebral embolism [in French]. Rev Neurol (Paris) 1995; 151: 583–5.