A 60-year-old female presented with confusion. Unable to give a coherent history, she was disoriented in time, with perseverative speech with confabulation and disinhibition. Collateral history indicated progressive decline over 4 years with inability to self-care, an account that she disputed. There was no family history of similar problems. On ACE-III she scored 32/100 (attention 6/18, memory 3/26, fluency 4/14, language 9/26, visuospatial 10/16). A provisional diagnosis of behavioural variant frontotemporal dementia (bvFTD) was made.
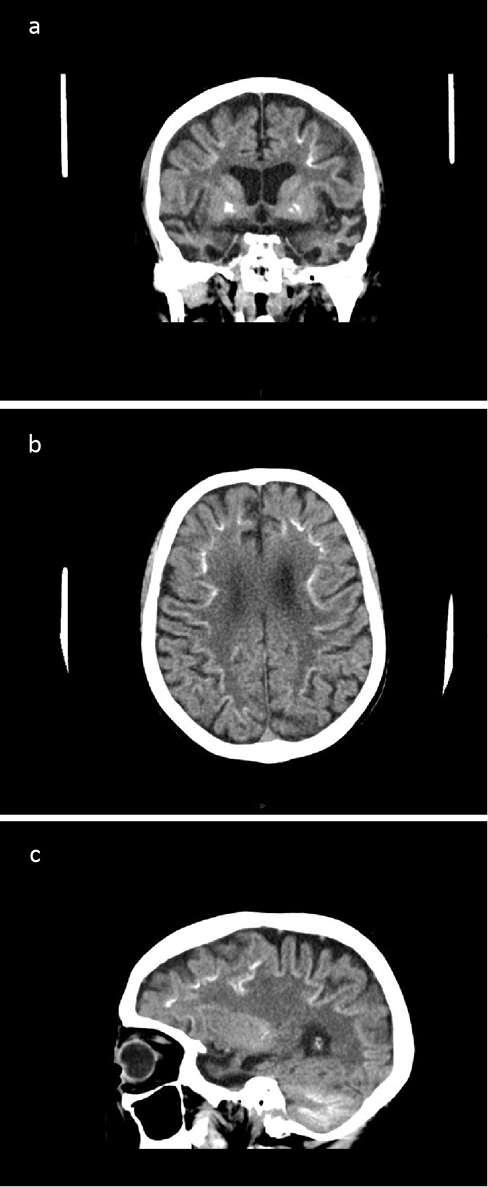
CT brain showed significant bilateral temporal lobe atrophy (Figure 1a, c). In addition, bilateral calcification of the basal ganglia was evident (Figure 1a) with linear calcification at the grey–white matter junction within both cerebral hemispheres (Figure 1b, c). Extensive calcification was also seen in the cerebellar folia (Figure 1c). MR brain imaging confirmed marked bilateral temporal lobe atrophy.
Figure 1 CT brain in (a) coronal, (b) axial and (c) sagittal views showing (a, c) cerebral atrophy particularly of the temporal lobes, calcification of (a) basal ganglia, (b, c) grey–white matter junction within the cerebral hemispheres and (c) cerebellar folia
The imaging findings of widespread calcification prompted investigation of indices of calcium metabolism. Calcium, phosphate and parathyroid hormone (PTH) were all normal. The clinical features and focal temporal lobe atrophy prompted neurogenetic testing for FTD. C9orf72 hexanucleotide repeat expansion was negative as was next-generation sequencing of a panel of dementia-related genes (including tau, progranulin, VCP, CHMP2B, TREM2 and CSF1R).
Dementia associated with brain calcification may occur in Down’s syndrome and some cases of Fahr’s disease (bilateral striatopallidodentate calcinosis). Basal ganglia calcification may occur in Nasu–Hakola disease resulting from TREM2 mutations, and punctate calcification of subcortical and deep white matter may occur in adult-onset leukodystrophy with axonal spheroids and pigmented glia resulting from CSF1R mutations. Brain calcification in hypoparathyroidism or pseudohypoparathyroidism may occasionally be associated with cognitive impairment. All these diagnostic possibilities were excluded by the clinical and investigation findings.
Chance concurrence of two separate disorders (dual pathology), FTD and idiopathic brain calcification, might explain this case. However, a plausible unifying diagnosis for this phenotype is Kosaka–Shibayama disease, or diffuse neurofibrillary tangles with calcification (DNTC). This rare disorder of unknown aetiology is reported almost exclusively from Japan.1 The clinical phenotype may suggest either Alzheimer’s disease or FTD, with characteristic temporal or temporofrontal atrophy. Although the evidence base is limited (<50 cases reported), this appears to be a nonfamilial presenile disorder more common in females. Suggested diagnostic criteria were fulfilled: essential (dementia), three core features (basal ganglia calcification, temporofrontal syndrome, localised atrophy of temporal lobes), as well as four of the six supportive features (presenile onset, lack of insight into disease, loss of initiative, normal serum levels of calcium, phosphate and PTH).
Only one case of DNTC has previously been reported from the UK,2 based on pathological findings without evidence of dementia. It is likely that other cases of Kosaka–Shibayama disease have been labelled as having sporadic Fahr’s disease.3
References
1 Ukai K, Kosaka K. Diffuse neurofibrillary tangles with calcification (Kosaka-Shibayama disease) in Japan. Psychiatry Clin Neurosci 2016; 70: 131–40.
2 Langlois NE, Grieve JH, Best PV. Changes of diffuse neurofibrillary tangles with calcification (DNTC) in a woman without evidence of dementia. J Neurol Neurosurg Psychiatry 1995; 59: 103.
3 Modrego PJ, Mojonero J, Serrano M et al. Fahr’s syndrome presenting with pure and progressive presenile dementia. Neurol Sci 2005; 26: 367–9.
