Introduction
Rabies is the most fatal viral infection known, with a mortality rate of 100%. According to the World Health Organisation (WHO), rabies is considered as a neurological infectious neglected tropical disease (NTD). Ninety-five per cent of global cases are within Asia and Africa (Figure 1).1 Nevertheless, despite its high fatality rate, rabies is thought to be potentially preventable with good public health measures and policies.
Figure 1 World map showing high-risk regions for rabies (highlighted). Sibu is shown in the magnified view of Borneo. Modified based on the WHO world map for rabies 2013.5 In 2013, Malaysia had been declared rabies free.
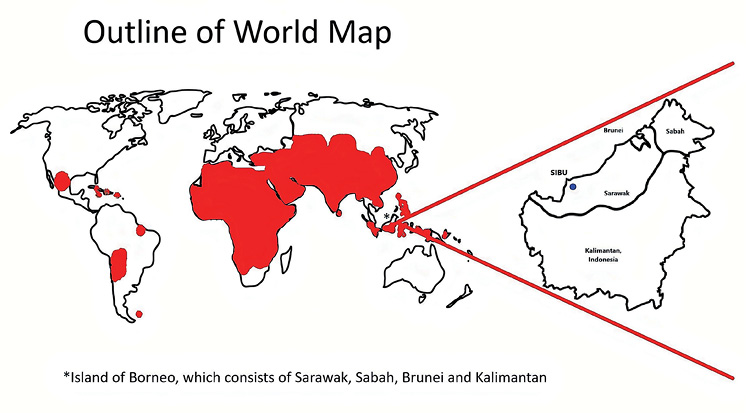
Sarawak, which is part of the island of Borneo, is the largest state in Malaysia, with an estimated population of 2.9 million in 2020.2 A rabies outbreak was declared in Sarawak in 2017 and it was believed that the virus might have originated from southern Kalimantan, where rabies has been endemic since 1906.3 Since 2017, the number of rabies cases and deaths have skyrocketed, totalling 29 fatalities at end of 2020. The first ever case of rabies in Sibu, a district in Sarawak, was recorded in March 2020, involving a young girl who was bitten by a rabid stray dog on her face. In a recent statement by the local government, it was revealed that nearly half of the dogs sampled in Sibu had the rabies virus. Rabies is now considered an evolving epidemic in Sarawak.4
We summarise the rabies cases encountered at our centre and highlight the factors linked to their mortality. Further, we formulated an algorithm based on our clinical observations to guide the diagnosis of rabies via clinical phenotypic recognition, particularly in resource-limited healthcare settings.
Methods
Admission notes were traced from the Medical Record Unit of Sibu Hospital based on recalls by the treating doctors for the period between March 2020 and February 2021. Cases of confirmed and probable rabies recorded in our centre over the one-year period were identified based on the WHO case definition for rabies.1
A ‘probable’ rabies is defined by a suspected case from an endemic area, presenting with progressive, fatal encephalitis accompanied by a positive history of contact with rabid animals. A ‘definite’ rabies case also fulfils these criteria, but with laboratory confirmation of either the presence of positive rabies nucleic material via polymerase chain reaction (PCR) or autopsy findings confirming rabies.1
Encephalitis cases with an alternative diagnosis and/or that follow a non-progressive clinical course were excluded from our search. By considering the inclusion and exclusion criteria, we managed to identify six cases of rabies (five confirmed and one probable) for our study.
Approval from the patients’ next-of-kin and ethical approval were deemed not required because the patients’ confidentiality and identities are preserved in this study, after consultation with our local research committee.
Results
A summary of the six cases of rabies is shown in Table 1. We identified six cases during the one-year period, which consisted of five confirmed cases and one probable case. The age of our subjects ranged from the youngest at 4 years old to the oldest at 62 years old. Most of our subjects were female (four out of six). Five subjects did not seek medical attention after the bite incident, and were therefore denied early appropriate treatment, which includes wound washing, rabies vaccination and rabies immunoglobulin administration. The incubation period for our cases also varied widely from 17 days to 2 years. In 2020, the cumulative incidence of rabies in Sibu was calculated as 1.7 per 100,000 population, with an estimated Sibu population of 350,000.2
Table 1 Summary of the rabies cases encountered in Sibu Hospital from March 2020 to February 2021 (N/A, not available)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1
|
4
|
Female
|
Yes, by rabid stray dog on the face
|
X
|
X
|
Yes
|
17 days
|
Furious
|
Both
|
3 days
|
X
|
Definite rabies
|
|
2
|
62
|
Male
|
Yes, by rabid stray dog on the right leg
|
X
|
X
|
X
|
5 weeks
|
Paralytic
|
Both
|
8 days
|
X
|
Definite rabies
|
|
3
|
20
|
Female
|
X
|
N/A
|
N/A
|
N/A
|
N/A
|
Both
|
Both
|
19 days (supported in ICU)
|
Yes. Signal abnormalities at bilateral basal ganglia, thalamus, midbrain, hypothalamus, cervical cord, diffuse enhancement
|
Probable rabies (rabies PCR not available)
|
|
4
|
34
|
Female
|
Yes, by rabid stray dog on the right leg
|
X
|
X
|
X
|
2 years
|
Paralytic
|
Both
|
12 days
|
Yes. Signal abnormalities at medulla, spinal cord, conus medullaris & lumbosacral roots from L2–S2
|
Definite rabies
|
|
5
|
53
|
Female
|
Yes, by rabid pet dog on the left hand
|
X
|
X
|
X
|
2 months
|
Furious
|
Both
|
11 days
|
Yes. Normal MRI brain
|
Definite rabies
|
|
6
|
52
|
Male
|
Yes, by rabid, stray dog on the right hand
|
X
|
X
|
X
|
5 months
|
Furious
|
Both
|
12 days
|
Yes. Normal MRI brain
|
Definite rabies
|
In terms of clinical phenotypes upon presentation to the health facility, four subjects manifested the furious (encephalitis) variant, whereas two other cases presented with the paralytic type. Encephalitic rabies is defined by the presence of delirium, confusion, aerophobia, hydrophobia, brainstem signs, pharyngeal spasm and/or coma. Paralytic rabies exhibits progressive weakness of the limbs, which may be ascending in nature, mimicking Guillain-Barré Syndrome. As the disease progressed, and prior to death, all the subjects displayed mixed phenotypes with a combination of upper and lower motor neuron involvement.
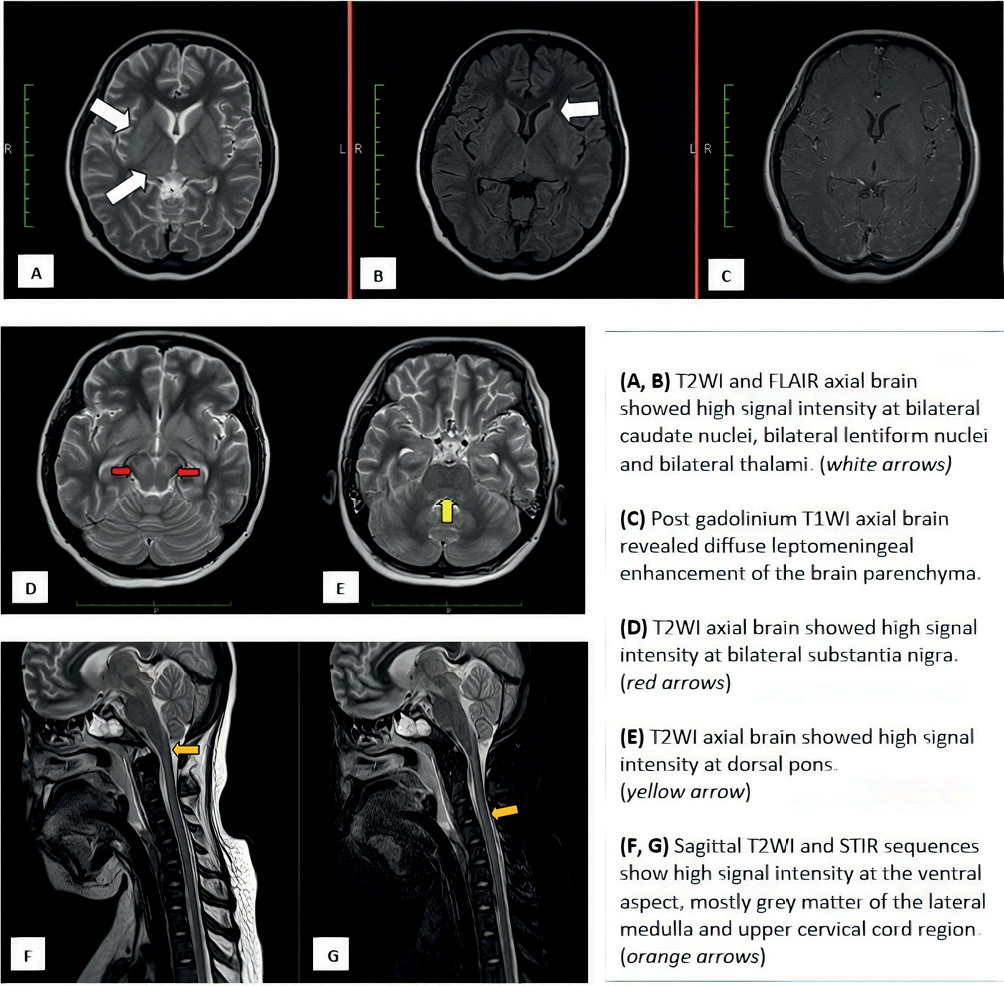
There was no survival in our study. Five cases succumbed to the illness within 14 days from symptom onset. One case (Case 3 with probable rabies) survived for 19 days as she was placed on ventilator support, while actively searching for an underlying aetiology. Unfortunately, rabies PCR was not carried out for her because of the lack of history of rabid animal contact. Her history was interrogated extensively via her family members because she was comatose. Her family members strongly denied any history of dog bite or bite from other potential vectors like cats. Nevertheless, she was suspected to have probable rabies as she had a typical history of progressive encephalitis (rhombencephalitis, autonomic dysfunction and coma) with supporting imaging findings pathognomonic for rabies rhombencephalitis (Figure 2). Common causes for rhombencephalitis, like Japanese encephalitis, Listeria monocytogenes, herpes simplex virus and enterovirus were excluded in her case.
Figure 2 Imaging features for Case 3
Five of the six subjects had rabies PCR carried out on their urine, cerebrospinal fluid, skin and saliva, which subsequently came back as positive for rabies. The specimens were sent to the University Malaysia Sarawak Research Laboratory and the Malaysian Ministry of Health Institute of Medical Research Laboratory, Kuala Lumpur for analysis.
Magnetic resonance imaging (MRI) of the central nervous system was conducted for four subjects. Of the four subjects, two subjects revealed imaging abnormalities, while the remaining two showed unremarkable changes. The abnormalities seen for Case 3 (furious type) were at the basal ganglia, thalamus, midbrain, hypothalamus and cervical cord with diffuse cortical enhancement. For Case 4 with paralytic type, the abnormalities were noted at the medulla, spinal cord, conus medullaris and lumbosacral roots from L2 to S2 level. There was no imaging performed for Cases 1 and 2 because of their classical presentation with obvious history of contact with rabid dogs.
Discussion
Our study revealed that four out of the five cases with a history of dog bite did not seek medical attention immediately after the bite. We interviewed family members regarding the history of dog bite, but most were initially unable to provide any history of contact with rabid dogs. Further history was only obtained after contacting other family members and persistently enquiring about any animal bite history. To our surprise, the families of all cases did not know the importance of seeking prompt medical attention after a dog bite.
We learned that the lack of bite history does not necessarily rule out the possibility of rabies because minor wounds may often be disregarded by adults without the knowledge of their family members. A history of dog bite should be scrutinised, and rabies PCR should be carried out if there is strong clinical suspicion and phenotypic recognition from a collection of symptoms, even when this history is unavailable.
Worldwide, 90% of rabies transmission results from contact with the saliva of a rabid dog, mostly from bites.1 Other animals reported to transmit rabies includes bats, raccoons, skunks and foxes.6 In Sarawak, positive rabies viral PCR has been isolated from dogs and cats, although all confirmed cases of human rabies have been from dog bites.7
Parents are more conscious about dog bites involving their children and tend to seek treatment earlier. Case 1 presented to a healthcare facility shortly after sustaining multiple deep laceration wounds over her face and the oral mucosa. However, wound washing was not initiated immediately by her parents. This child was given a full dose of rabies immunoglobulin within 24 hours of the bite and completed the scheduled vaccination. Unfortunately, she succumbed to the illness by day 17 of the bite, despite early post-exposure prophylaxis (PEP). This shows that PEP alone does not guarantee protection if early management of the bite wound is not properly initiated to reduce the load of inoculated virus, particularly at sites closest to the brain.
These observations are worrying as they reflect the lack of awareness among the public in relation to dog bites and immediate management, which can reduce the risk of death significantly when done promptly and correctly. A few studies in Asia (Malaysia not included) have revealed that the rabies awareness among the Asian population was low and there was delay in getting the appropriate treatment.8,9 Unfortunately, there is no similar study in Malaysia, particularly in Sarawak. We hope that our study may encourage more effort in conducting epidemiological surveillance and primary prevention within our local setting.
The incubation period for rabies is typically between one and three months.1 However, the incubation could be shorter (17 days for Case 1) or longer (two years for Case 4), as seen in our cases. There is no exact scientific explanation for this wide discrepancy in incubation period, although various factors such as the site of inoculation, infectivity of the rabid animal at the time of the bite and the immune status of the host may play some role.10
In our approach, we emphasised the detailed history, identified the clinical phenotype and pursued rabies work-up in cases that were strongly suspected. We realised that all our cases did not follow the clear distinction between the encephalitis and paralytic variant, as reported in the literature, and tended to present with a mixed phenotype, especially during the advanced stage. Although neuroimaging may be a useful adjunct to the diagnosis, the diagnostic yield is low. The neuroimaging findings are often unremarkable, especially during the early stages. The odds for neuroimaging abnormalities increase with serial imaging as the disease progresses, as described by Rao et al.11 Imaging abnormalities described in rabies include T2 signal grey matter abnormalities with predilection for basal ganglia, thalamus, hypothalamus, brainstem, spinal cord and nerve roots.12 With a typical rabies presentation, neuroimaging can be exempted from investigations. Neuroimaging may help in cases when the presentation is either atypical for rabies or other diagnosis could not be excluded. For instance, MRI can distinguish acute disseminated encephalomyelitis from rabies because the former rarely involves grey matter.13
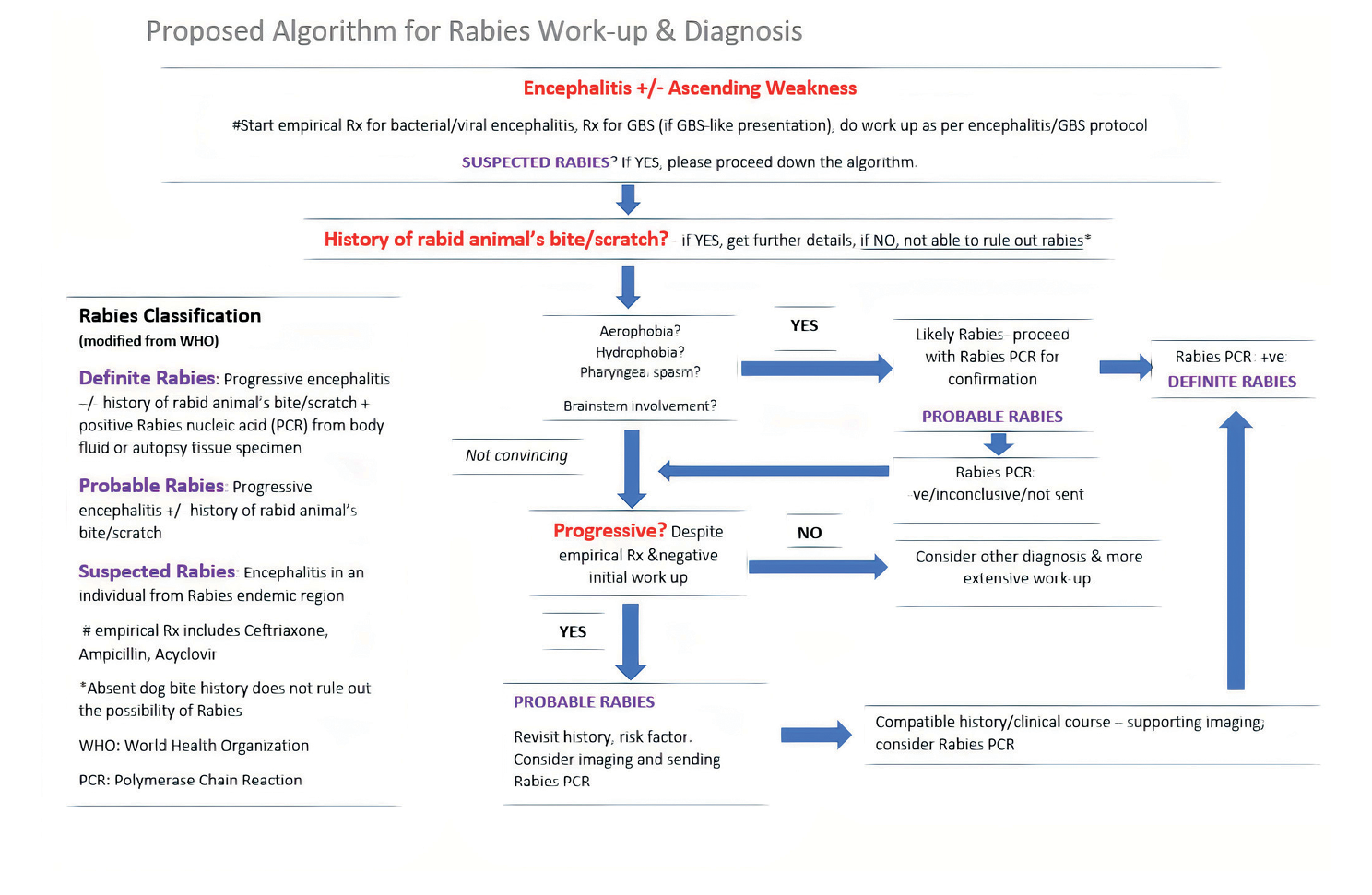
Figure 3 illustrates our proposed algorithm for rabies work-up and diagnosis. We have incorporated the WHO rabies classification (suspected, probable, and definite) into the algorithm, as this may reflect the level of certainty for rabies diagnosis. We recommend that empirical treatment and initial investigations should be instituted at an early stage, depending on the patient’s clinical presentation. Progressive encephalitis, especially with features of rhombencephalitis with dysautonomia, was occasionally preceded by lower motor neuron flaccid paralysis (like Guillain-Barré Syndrome) without a known aetiology, and remains the most important distinguishing characteristic of rabies from other encephalitis aetiologies. It is our hope that this algorithm will help clinicians in resource-limited settings to have a more targeted, pragmatic approach in their management of suspected rabies.
Figure 3 Diagnostic and work-up algorithm for rabies
Despite recent advancements in the treatment of other types of encephalitis, there is still no effective treatment for rabies. In 2005, Ribavirin, Amantadine and Ketamine were used to induce burst suppression for cases with human rabies, with the aim of giving time for the body’s immune system to mount a defence against the virus. However, this protocol, known as the ‘Milwaukee’ protocol, did not stand against the test of time and was later found to be ineffective for treating rabies.14 Once rabies is confirmed, the treatment is only supportive, as is seen in our cases. Given the treatment futility, prevention remains to be the only effective option.
Rabies remains the deadliest, yet potentially preventable, neurological infection known. The patient’s history of rabid animal contact may not be available, and the incubation period can vary. The cumulative incidence for rabies in our local population (1.7 per 100,000 population) is probably an underestimation. Therefore, a strong clinical suspicion and low threshold to send for rabies work-up should always be considered. We hope that our rabies series may encourage future studies because prevention is more important that diagnosis and treatment for this NTD. 
Acknowledgements
The authors would like to thank the Director General of Health Malaysia for the permission to publish this paper. We would also like to express our gratitude to the hospital director of Sibu Hospital and all the healthcare providers, including the infectious disease team of Sarawak General Hospital for their valuable tele-consultation.
