Introduction
Meningitis is a clinical syndrome characterised by inflammation of the meninges surrounding the brain and the spinal cord. The classic triad of meningitis consists of fever, headache and neck stiffness.1 Meningitis can be of infective or noninfective aetiology, occurring in any age group, with extremes of age being the most severely affected. The immune-compromised state also has high mortality and morbidity. The overall case fatality rate of bacterial meningitis in adult patients is around 30%.2–4
Although encephalitis by definition involves the brain parenchyma, it may also involve the meninges as well, which is termed as ‘meningoencephalitis’. From an epidemiologic and pathophysiologic perspective, encephalitis is distinct from meningitis, though on clinical evaluation both can coexist. The clinical presentation is encephalopathy with diffuse or focal neurological symptoms, including behavioural and personality changes, decreased level of consciousness, neck pain/stiffness, photophobia, lethargy, generalised or focal seizures, acute confusion or amnesic states, and flaccid paralysis.5
Organisms responsible for bacterial meningitis are Streptococcus pneumoniae, Neisseria meningitis, Group B streptococci, Listeria monocytogens and Haemophilus influenza type b. Most patients recover completely if appropriate antibiotic therapy is instituted promptly. Mycobacterium tuberculosis is another major cause, especially in developing countries. Tuberculous meningitis is a critical disease in terms of fatal outcome and permanent sequelae, requiring rapid diagnosis and treatment.6
The term aseptic meningitis is used for all types of inflammation of the brain meninges not caused by pus-producing bacteria. It is usually a benign syndrome. Viral and aseptic meningitis are terms used interchangeably as, not only viruses are a major cause, other noninfective causes are equally attributable to the development of meningitis. Worldwide causes of viral meningitis include enterovirus, herpes, mumps, measles and HIV, with enterovirus being the most common cause of viral meningitis.
In the emergency setting differentiating bacterial meningitis from other causes, such as fungal, tuberculous, viral, neoplastic, toxic or autoimmune causes, is extremely difficult. If a diagnosis of meningitis is made, it is prudent to start the patient on empirical antibiotics until the cultures and other results are awaited.7 Whereas plenty of literature is available on meningitis, the clinico-aetiological profile and outcome in patients with meningoencephalitis remains not that well studied, except for in the paediatric population.8 The present study was carried out to assess the causes, clinical profile and outcome in adults diagnosed with meningoencephalitis in a tertiary care hospital.
Methods
Patients
The study was based on prospective collection of data in adults aged 18 years and above who were diagnosed with meningoencephalitis of various aetiologies, confirmed with laboratory investigations and brain imaging. The study was conducted in a tertiary care centre, Coimbatore, South India, from July 2014 to July 2015.
Inclusion and exclusion criteria
Patients with community-acquired meningoencephalitis, those who underwent lumbar puncture for the diagnosis and those with both positive and negative cerebrospinal fluid cultures were included in the study. Patients with hospital-acquired meningitis, patients with myelitis, neurosurgical devices or a recent history of neurosurgery were excluded.
Assessments
Data were extracted using a proforma that included detailed history, clinical examination details and requisite investigations. History and clinical findings attributable to the meningitis and meningoencephalitis were collected in detail, which included fever, headache, vomiting, neck stiffness, seizures, altered sensorium, cerebrospinal fluid (CSF) rhinorrhoea and focal neurological deficits. In addition to basic investigations (which included complete blood count, blood sugar, renal and liver function tests, and chest X-ray), blood culture was also taken for all patients. All underwent lumbar puncture and CSF analysis was carried out that included sugar, protein, total count, differential count, Gram stain, bacterial culture, Ziehl Neelsen stain for acid-fast bacilli (AFB), India ink stain for Cryptococcus, cryptococcal antigen test and automated culture for AFB. Neuroimaging (CT or MRI brain) was carried out on all patients. CSF polymerase chain reaction (PCR) was carried out in selected patients. Herpes simplex virus (HSV) PCR was carried out in selected patients with suspected HSV encephalitis based on clinical, CSF and neuroimaging findings.
Based on the duration of illness, patients were classified into acute (<1 week), subacute (1–4 weeks) and chronic (>4 weeks) and also grouped based on aetiology. To assess the clinical outcome of the patients the Barthel index was used.9 Barthel index is an ordinal scale used to measure the performance in 10 activities of daily living with the score ranging between 0 and 20; functional evaluation was carried out at the end of the first month. A score of <12 is taken as a poor outcome and >12 as a good outcome; death is included in the poor outcome category. The results were analysed to assess the clinical presentation, aetiology and clinical outcome.
Ethical clearance
The study was approved by the Institutional Human Ethics Committee (Approval Number 14/193).
Results
The study included 50 consecutive patients with acute meningoencephalitis. Among 50 patients, 33 (66%) were male, 17 (34%) were female. The majority of them (78%) were <50 years of age (Table 1).
Table 1 Age-wise distribution of the study subjects
| | | |
18–30 | 12 | 5 | 17 |
31–50 | 17 | 5 | 22 |
51–60 | 2 | 7 | 9 |
>60 | 2 | 0 | 2 |
Total | 33 | 17 | 50 |
Clinical presentation
The common initial presenting symptoms were fever, headache and altered sensorium (Table 2). Among 50 patients, 32 (64%) had both fever and headache; only 18 (36%) had all the three classical signs of meningitis – headache, fever and altered sensorium. Twenty-eight patients (56%) did not have neck stiffness, even though there was meningeal involvement as noted in neuroimaging. Six patients (12%) had seizures; five had generalised tonic–clonic seizures, while focal motor seizures was noted in one. Among those six patients, one had a parenchymal lesion in the MRI (granuloma), meningeal enhancement was noted in three and MRI was normal in two.
Table 2 Clinical presentation of the study subjects
| | |
Headache | 37 | 74 |
Fever | 41 | 82 |
Seizures | 6 | 12 |
Vomiting | 24 | 48 |
Altered mental status | 31 | 62 |
Cerebrospinal fluid rhinorrhea | 5 | 10 |
Hemiparesis | 4 | 8 |
Speech disturbances | 2 | 4 |
Cranial nerve palsy | 4 | 8 |
Neck stiffness | 22 | 44 |
Papilloedema | 5 | 10 |
Altered sensorium was observed in 31 patients; of which 10 had significant deterioration in consciousness (Glasgow coma scale <10). Among those with poor Glasgow coma scale, one had pyogenic meningitis with extensive vasculitic infarcts in the bilateral cerebral hemisphere and brainstem; seven had abnormal findings in MRI like meningeal enhancement, granuloma or hydrocephalus; MRI was normal in the remaining two. All of these 10 patients with altered sensorium required ventilatory support in intensive care unit. See online for Supplementary Tables 1–4.
Aetiology and mode of onset
The aetiological profile of meningoencephalitis is listed in Table 3. The most common cause found in the present study was M. tuberculosis. Among eight patients with acute pyogenic meningitis, the microbiological diagnosis was confirmed in five; three had pneumococcal growth in CSF analysis, while blood culture was positive in two (S. aureus; pneumococci). The remaining three patients with acute pyogenic meningitis had sterile cultures due to prior antibiotic exposure. However, very high neutrophil count in the CSF suggested the diagnosis of acute pyogenic meningitis.
Table 3 Aetiological profile of meningoencephalitis
| | |
Tuberculous meningitis | 29 | 58 |
Acute pyogenic meningitis | 8 | 16 |
Viral meningoencephalitis | 8 | 16 |
Cryptococcal meningitis | 2 | 4 |
Cerebral toxoplasmosis | 2 | 4 |
Aspergillus meningitis | 1 | 2 |
Total | 50 | 100 |
Acute and subacute clinical presentation was seen in 23 each while the remaining four presented with chronic symptoms. The mode of onset of symptoms in various aetiologies is listed in Table 4.
Table 4 Mode of onset of symptoms in various aetiologies of meningoencephalitis
| | | | |
Tuberculous meningitis | 4 | 21 | 4 | 29 |
Acute pyogenic meningitis | 8 | 0 | 0 | 8 |
Viral meningoencephalitis | 8 | 0 | 0 | 8 |
Cryptococcal meningitis | 1 | 1 | 0 | 2 |
Cerebral toxoplasmosis | 2 | 0 | 0 | 2 |
Aspergillus meningitis | 0 | 1 | 0 | 1 |
Total | 23 | 23 | 4 | 50 |
Outcome
Clinical outcome was assessed at the end of 1 month using the Barthel index. Among 50 patients, 47 (94%) recovered completely without any deficits; two had fulminant course and succumbed to the illness by the end of 1 month; one had Barthel index score of 0 (Table 5).
| | | | | | | |
Good (n = 47) | 29 | 6 | 8 | 1 | 2 | 1 | 47 |
Poor (n = 3) | 0 | 2 | 0 | 1 | 0 | 0 | 3 |
Total | 29 | 8 | 8 | 2 | 2 | 1 | 50 |
Discussion
The present study included 50 adult patients with meningoencephalitis, M. tuberculosis was the most common agent. Tuberculosis still poses a huge health threat in developing countries like India despite advances in the detection and management of the disease.
The common presenting symptoms were fever, headache and altered sensorium. This was similar to other studies with acute bacterial meningitis.2,10,11 In the present study, nine did not have fever (five had tuberculous aetiology, two had cryptococcal meningitis, cerebral toxoplasmosis and Aspergillus meningitis in one each). All patients with acute pyogenic and viral meningoencephalitis presented with fever. Only 18 out of 50 patients (36%) had all the three symptoms – fever, headache and neck stiffness; a similar observation (41%) was noted in a prospective cohort study of community-acquired bacterial meningitis in adults in the Netherlands.11 Neck stiffness was absent in 28 patients (56%), which indicates that absence of fever or neck stiffness does not exclude the possibility of infectious meningoencephalitis.
Diagnosis of pyogenic meningitis was made based on typical CSF findings (Supplementary Table 1) that showed high protein, low sugar and very high neutrophil count in the CSF (>1,000 cells/mm3 in most of the patients). In five out of these eight patients with acute pyogenic meningitis, organisms could be isolated either in the blood or CSF. For the remaining three, the cultures were sterile possibly due to prior antibiotic exposure. In a retrospective study of 305 patients with acute bacterial meningitis, 53 (17.4%) had received antibiotics before admission and among these, death occurred only in one. The death rate was high (12%) in the remaining 252 patients who had not received antibiotics before admission. This study indicates the importance of the early institution of antibiotic therapy.12,13
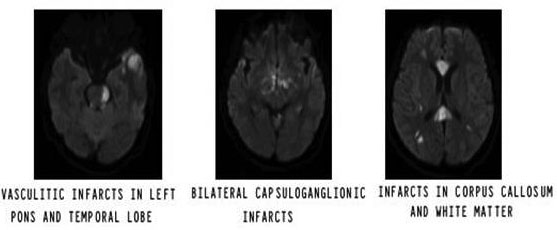
Surprisingly, four out of eight patients (50%) with acute pyogenic meningitis had a history of CSF rhinorrhoea before the onset of illness. Of these, three had a head injury in the past. MRI brain in one patient revealed a defect in the anterior cranial fossa that was the cause of CSF leak. One of them had recurrent episodes of pyogenic meningitis due to persistent CSF leak through the nose. After recovery from meningitis, he underwent a neurosurgical procedure for the correction of bony defects. This highlights the importance of elicitation of history for CSF rhinorrhoea in subjects with recurrent meningitis so that the underlying cause can be corrected. Six out of eight acute meningoencephalitis patients recovered completely without residual deficits; however, two had fulminant pneumococcal meningitis, of which one succumbed to the illness although treatment was initiated appropriately, the other survived with severe neurological disability, i.e. bilateral conjugate gaze paralysis of eyeballs and quadriparesis due to multiple infarcts (Figure 1). These outcomes highlight the fact that despite early diagnosis and appropriate treatment some patients with acute pyogenic meningitis may have a fulminant course with a severe neurological disability that can impair the quality of life.
Figure 1 Fulminant pneumococcal meningitis with extensive vasculitic infarcts in bilateral cerebral hemisphere and brainstem
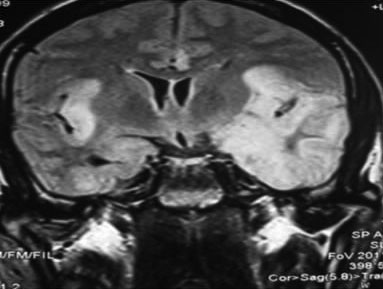
Eight patients had acute viral meningoencephalitis, diagnosed based on CSF analysis and brain imaging features. A viral aetiology was considered when CSF had slightly elevated protein, low-to-normal blood sugar and moderately elevated lymphocyte count. Bacterial cultures, Gram stain, AFB stain and India ink stain were negative in these patients. Out of these eight patients, microbiological confirmation (positive HSV PCR in CSF) was possible in only two; both had temporal cortex hyperintense signals in MRI brain (Figure 2). All recovered completely with acyclovir and were asymptomatic during follow up. In many cases of presumed viral encephalitis (32–75%), the responsible organism could not be pin-pointed despite detailed diagnostic testing. In the California Encephalitis Project, 334 patients with encephalitis were studied from 1998 to 2000; aetiology could not be identified in 208 (62%), despite extensive testing and evaluation.14
Figure 2 Herpes simplex encephalitis – T2-weighted MRI shows left temporal and right insular hyperintensity
Although commonly tuberculous and cryptococcal meningitis presents with subacute courses, four patients with tuberculous meningitis and one with cryptococcal meningitis presented with acute onset illness. Two patients presented with central nervous system toxoplasmosis and these patients were found to be HIV positive; they presented with hemiparesis; CSF serology for toxoplasma immunoglobulin G was positive; both the patients recovered well with treatment.
In the present study 15 had basal meningeal exudates on brain imaging. Hydrocephalus was present in five; of which two had significantly larger hydrocephalus, which progressively increased in size and both underwent ventriculoperitoneal shunt. In two large community-based series, hydrocephalus was seen in approximately 75% of patients, basilar meningeal enhancement in 38%, cerebral infarcts in 15–30% and tuberculomas in 5–10%.15,16 With early appropriate antituberculous therapy and steroids, all patients with tuberculous meningitis in the present study recovered well without neurological deficits.
The present study had several limitations: PCR studies in all CSF culture-negative samples could not be performed and the study was conducted in a single centre with a limited number of patients. Further larger studies on meningoencephalitis would throw more light on the clinical profile and outcome.
In conclusion, the present study underscores the fact that the absence of fever or neck stiffness does not exclude the possibility of infectious meningoencephalitis. The focus in all cases should be on early diagnosis and appropriate treatment, though in some patients the outcome may not be uniformly good.
Online Supplementary Material
Supplementary Tables 1–4 are available with the online version of this paper, which can be accessed at https://www.rcpe.ac.uk/journal.
References
1 Ginsberg L, Kidd D. Chronic and recurrent meningitis. Pract Neurol 2008; 8: 348–61.
2 Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med 1998; 129: 862–9.
3 Mace SE. Acute bacterial meningitis. Emerg Med Clin North Am 2008; 26: 281–317.
4 Roos KL, van de Beek D. Bacterial meningitis. Handb Clin Neurol 2010; 96: 51–63.
5 Bloch KC, Glaser C. Diagnostic approaches for patients with suspected encephalitis. Curr Infect Dis Rep 2007; 9: 315–22.
6 Rich AR, McCordick HA. The pathogenesis of tuberculous meningitis. Bull John Hopkins Hospital 1933; 52: 5–37.
7 Fitch MT, Abrahamian FM, Moran GJ et al. Emergency department management of meningitis and encephalitis. Infect Dis Clin North Am 2008; 22: 33–52.
8 Khajeh A, Sharifi-Mood B, Soleimani GR. Pediatric meningoencephalitis; a research on patients hospitalized in Zahedan, Southeastern Iran. Int J Infect 2015; 2: e23835.
9 Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965; 14: 61–5.
10 Van de Beek D, de Gans J, Spanjaard L et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351: 1849.
11 Bijlsma MW, Brouwer MC, Kasanmoentalib ES et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis 2016; 16: 39.
12 The Research Committee of the British Society for the Study of Infection. Bacterial meningitis: causes for concern. J Infect 1995; 30: 89–94.
13 Begg N, Cartwright KAV, Cohen J et al. Consensus statement on diagnosis, investigation, treatment, and prevention of acute bacterial meningitis in immunocompetent adults. J Infect 1999; 39: 1–15.
14 Glaser CS, Gilliam S, Schnurr D et al. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis 2003; 36: 731–42.
15 Bhargava S, Gupta AK, Tandon PN. Tuberculous meningitis–a CT study. Br J Radiol 1982; 55: 189.
16 Ozates M, Kemaloglu S, Gurkan F et al. CT of the brain in tuberculous meningitis. A review of 289 patients. Acta Radiol 2000; 41: 13.
