Introduction
Pneumocystis jirovecii (PJ), previously known as Pneumocystis carinii (PC), is a fungus that most commonly affects immunocompromised hosts. It is known to cause severe, life-threatening illness in patients with underlying disease states that alter host immunity, such as HIV, cancer, transplant recipients or those taking immunosuppressive therapies and medications.1 PJ has seldom been seen to cause significant infections in those with a competent immune system. PJ affects patients worldwide and is spread from person to person via an airborne route. Though the availability and extensive use of antiretroviral treatment and prophylaxis has minimised the incidence of Pneumocystis infection in patients with HIV infection, it still remains one of the most common causes of serious opportunistic respiratory tract infection in these patients.1 Extrapulmonary Pneumocystis infection may occur in HIV-infected patients receiving pentamidine prophylaxis and advanced HIV infection without prophylaxis. It has been reported to affect all organ systems, including the nervous system, marrow, lymph nodes, eye, gastrointestinal tract and thyroid.2 Only a few isolated reports of PJ infecting the adrenal glands have been reported, mostly in those with advanced HIV infection. Isolated extrapulmonary Pneumocystis infection in immunocompetent persons is extremely rare.
Case presentation
A 48-year-old male presented with complaints of low-grade fever, generalised weakness, significant loss of weight (nearly 10 kg) and decreased appetite for the last 9 months. There was no cough, abdominal pain, bladder or bowel complaints, lumps or swellings, chest pain, palpitations, headaches, visual impairment, night sweats, hyperpigmentation or paleness over the body. He was a diabetic for 4 years, on oral hypoglycaemic agents (metformin and glimepiride) with a fairly good control of blood sugar. There was no history of prior tuberculosis, surgery, drug abuse, blood transfusion or high-risk behaviours.
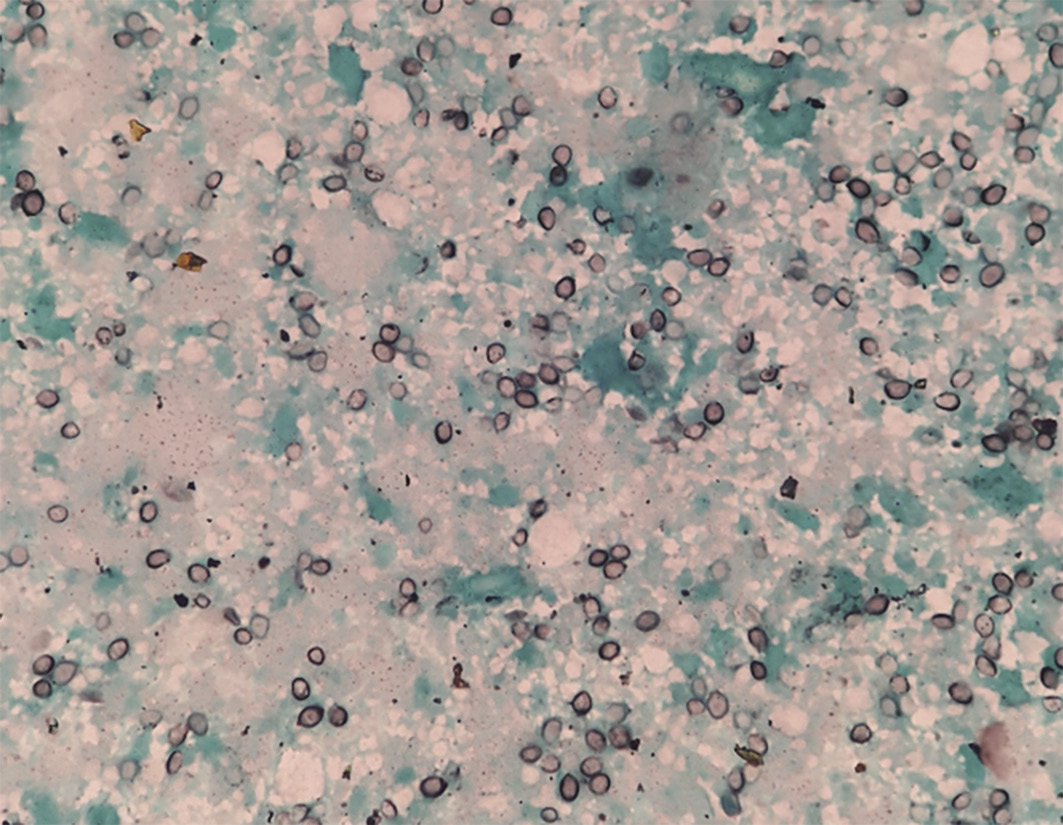
At presentation, his blood pressure was 80/60 mmHg and his Glasgow Coma Score was 15. There was no pallor, jaundice, cyanosis, clubbing, lymphadenopathy or rash. The detailed systemic examination was unremarkable. Blood investigations showed haemoglobin 14.4 g/dl, total leucocyte count 10,800/µl, platelet count 1.86 lac/µl, and erythrocyte sedimentation rate 32 mm in first hour. He had hyponatraemia (serum sodium 127 meq/dl), hyperkalaemia (serum potassium 6.4 meq/dl) and acute kidney injury (blood urea 98 mg/dl; serum creatinine 1.8 mg/dl). The chest X-ray, arterial blood gas analysis, coagulation profile and electrocardiogram were normal. Blood and urine cultures for both bacteria and fungus were negative. Serum cortisol levels were low (8 µg/dl) with normal serum renin, adrenocorticotropic hormone and aldosterone levels. These findings suggested adrenal insufficiency. An ultrasound of the abdomen revealed a 6.8 × 5.1 cm, well-defined, hypoechoic lesion in the left adrenal gland with no significant internal vascularity. MR abdomen was suggestive of bilateral bulky adrenal glands with well-defined mass lesions replacing both adrenal glands along with restricted diffusion with extensions and adjacent perihepatic collection to segment VII of right lobe of liver with multiple enlarged lymph nodes in the retroperitoneum and porta hepatis. A fluorodeoxyglucose PET scan showed heterogeneously hypermetabolic enlarged bilateral adrenal glands with periportal enhancement and central necrotic areas with no evidence of metabolically active lesion elsewhere in the visualised region of the body (Figure 1). Endoscopic ultrasound-guided fine-needle aspiration (FNAC) of left adrenal mass showed predominantly necrotic cells with interspersing negative shadowed, round, well-defined cyst-like structures. These cysts on Gomori methenamine silver (GMS) and Giemsa stain revealed presence of PJ (Figure 2). Gene Xpert-MTB (Cepheid, USA) of FNAC sample was negative. Aspirate culture did not show growth on Sabouraud dextrose agar or BACTEC™ (BD, USA) media after 4 weeks of incubation. Bacteriological cultures were also sterile. Hepatitis B and C, and HIV were negative. Serum IgA and IgG levels were in the normal range and CD4 count was 706/µl. There was no clinical or radiological evidence of Pneumocystis infection of the respiratory tract.
Figure 1 Fluorodeoxyglucose PET scan showing heterogeneously hypermetabolic enlarged bilateral adrenal glands with periportal enhancement and central necrotic areas (arrows)
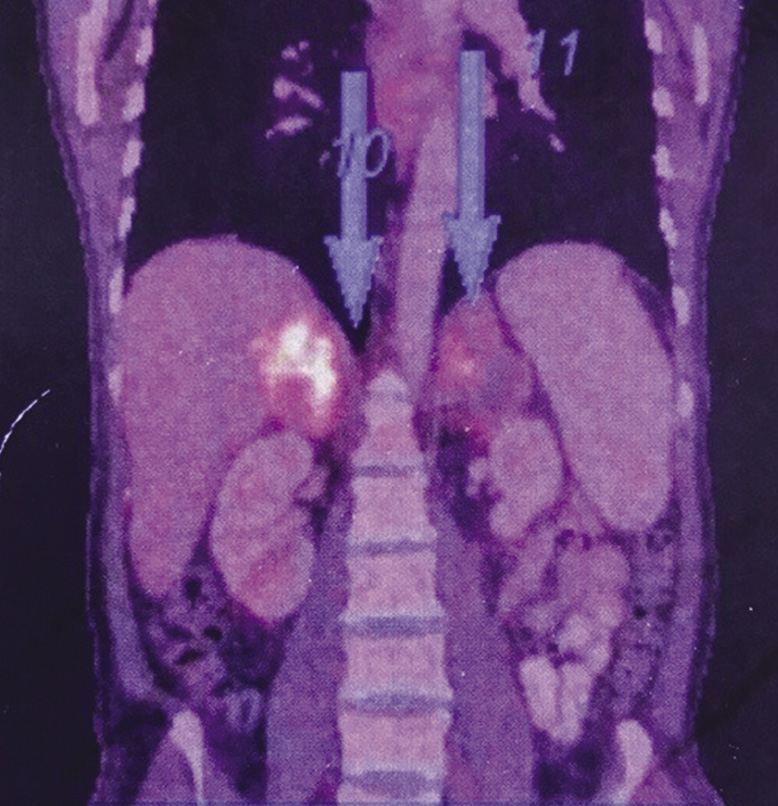
Figure 2 Fine-needle aspiration cytology of left adrenal mass showing predominantly necrotic cells with interspersing round, well-defined cysts of Pneumocystis jirovecii on Gomori methenamine silver stain (magnification × 100)
The patient was managed for adrenal crisis with steroid replacement. For PJ infection, he was initiated on double strength (DS) cotrimoxazole (trimethoprim/sulfamethoxazole: 160/800 mg), two DS tablets three times a day, and clindamycin (600 mg eight hourly) for 4 weeks. He improved symptomatically and was discharged on oral steroids. Patient was fully compliant to treatment.
He presented again in adrenal crisis after 5 months with fever, hyponatraemia, hyperkalaemia and hypotension. Repeat imaging performed was suggestive of increase in the size of right adrenal gland and nearly same size of left-sided adrenal lesion with new collections in the left hypochondrium (4.7 × 5.5 cm). A repeat FNAC of the adrenal mass again showed PJ infection.
In view of nonresponse to treatment, second-line treatment for PJ was started with primaquine and clindamycin for 4 weeks. Abdominal laproscopic examination performed post treatment revealed resolution of perihepatic and left hypochondrium collections. The patient improved after second-line treatment completion and was discharged on oral steroids. Patient is currently under follow up and doing well.
Discussion
Extrapulmonary PJ remains a rare cause of systemic infection even among HIV-infected patients. A few isolated case reports have been published on disseminated pneumocystosis in patients with altered immunity.3 To the best of our knowledge there exists only a single published case report of isolated adrenal infection due to PJ infection in an immunocompetent host.4
PJ pneumonia, earlier known as PC pneumonia (PCP), is a common opportunistic infection (OI) in patients with HIV infection, presenting with fever, cough, dyspnoea and respiratory failure. It was once estimated that approximately 66–85% patients infected with HIV-1 infection will have one episode of PCP in their lifetime.5 Owing to the widespread use of antiretroviral treatment and OI prophylaxis, the incidence has decreased dramatically globally. Extrapulmonary pneumocystosis is exceedingly rare. Most of these cases occurred at a time when pentamidine prophylaxis was extensively used in HIV OI prophylaxis. The overall incidence or prevalence of extrapulmonary pneumocystosis in immunocompetent individuals cannot be accurately estimated, as there are only a few published reports of such cases.
PJ can disseminate from the lungs to other organs, producing infection in almost any anatomic site, including lymph nodes, bone marrow, liver, kidneys, adrenal glands, spleen, gastrointestinal tract, brain, eyes and ear.5 It may have a broad spectrum of variable and nonspecific clinical presentations. PJ is rarely suspected as an initial diagnosis, owing to its low frequency of occurrence and these varied nonspecific symptoms can be attributed to a variety of other common infectious agents. PJ cannot be grown using current culture techniques. Demonstration of Pneumocystis cysts or trophozoites in the sample using stains, such as GMS, Giemsa or rapid Giemsa-like stains, confirms the diagnosis. Thus, histologic examination alone of affected tissues is sufficient for diagnosis.5
The adrenal gland may be infected by a number of organisms, which may lead to adrenal insufficiency. These include many pathogenic fungal infections, such as histoplasmosis, cryptococcosis and, rarely, coccidioidomycosis, blastomycosis and candidiasis. Tuberculosis is the most important infection affecting the adrenal glands.6
The treatment for extrapulmonary Pneumocystis infection remains comparable to that for PCP. Our patient was initially started on first-line treatment with DS cotrimoxazole tablets (trimethoprim/sulfamethoxazole). The patient relapsed after initial improvement prompting initiation of second-line treatment with primaquine and clindamycin to which the patient responded. Whether extrapulmonary Pneumocystis infections should be treated with primaquine initially itself remains debatable.
Conclusion
In conclusion, adrenal involvement by PJ, though rare, may occur in immunocompetent patients and has to be considered in the differential diagnosis of bilateral/unilateral adrenal masses presenting with adrenal insufficiency. A diagnosis requires a high index of suspicion, especially in immunocompetent patients who may have nonspecific symptoms, clinical signs, laboratory and radiological features. Clinical specimens must be sent for histopathology and fungal cultures for a definite diagnosis. What makes our case interesting and rare, besides the fact that our patient was immunocompetent, is that our patient had adrenal Pneumocystis infection in the absence of any evidence of pulmonary involvement, which is the usual portal of entry of the pathogen.
References
1 Truong J, Ashurst JV. Pneumonia, Pneumocystis (carinii) jirovecii. Treasure Island (FL): StatPearls Publishing; 2018.
2 Bennett NJ. Pneumocystis jiroveci pneumonia (PJP) overview of Pneumocystis jiroveci pneumonia. 2017. http://emedicine.medscape.com/article/225976-overview#aw2aab6b7 (accessed 27/02/19).
3 Karam MB, Mosadegh L. Extrapulmonary Pneumocystis jirovecii infection: a case report. Braz J Infect Dis 2014; 18: 681–5.
4 Agarwal J, Agarwal G, Ayyagari A et al. Isolated Pneumocystis carinii infection of adrenal glands causing Addison’s disease in a non-immunocompromised adult. Endocr Pathol 2001; 12: 87–91.
5 Ng VL, Yajko DM, Hadley WK. Extrapulmonary pneumocystosis. Clin Microbiol Rev 1997; 10: 401–18.
6 Upadhyay J, Sudhindra P, Abraham G et al. Tuberculosis of the adrenal gland: a case report and review of the literature of infections of the adrenal gland. Int J Endocrine 2014; 2014: 87603.
