Introduction
Lyme disease, first described in 1977 as the cause of an epidemic form of oligoarthritis, is now known to be caused by three pathogenic species of the spirochaete Borrelia burgdorferi sensu lato complex: Borrelia afzelii, Borrelia garinii and B. burgdorferi sensu stricto. Clinical manifestations classically follow the trajectory of early localised, early disseminated and late disease. Early localised disease is associated with the appearance of erythema migrans (EM), whilst early disseminated disease is characterised by multiple EM lesions due to spirochaetaemia, with or without neurologic and cardiac involvement. Late disease is typically associated with large joint oligoarthritis, polyneuropathies or other neurologic syndromes.
Case presentation
A 35-year-old Caucasian female presented to the infectious diseases department in Edinburgh, Scotland, with a 12-day history of fever, headache and severe myalgia, 15 days after a 2-week trip to New York state. Five days before admission she developed a rash over her abdomen that spread to her face and limbs. During her trip she walked in long grasses close to the Canadian border and was aware of one mosquito bite but no adherent ticks. She had taken appropriate pretravel advice, and denied any unwell contacts. Her past medical history was notable for a seronegative inflammatory arthritis managed with fortnightly injections of certolizumab [a poly(ethylene glycol)ylated recombinant humanised antibody Fab’ fragment against tumour necrosis factor-alpha (TNF-α)] and daily sulfasalazine.
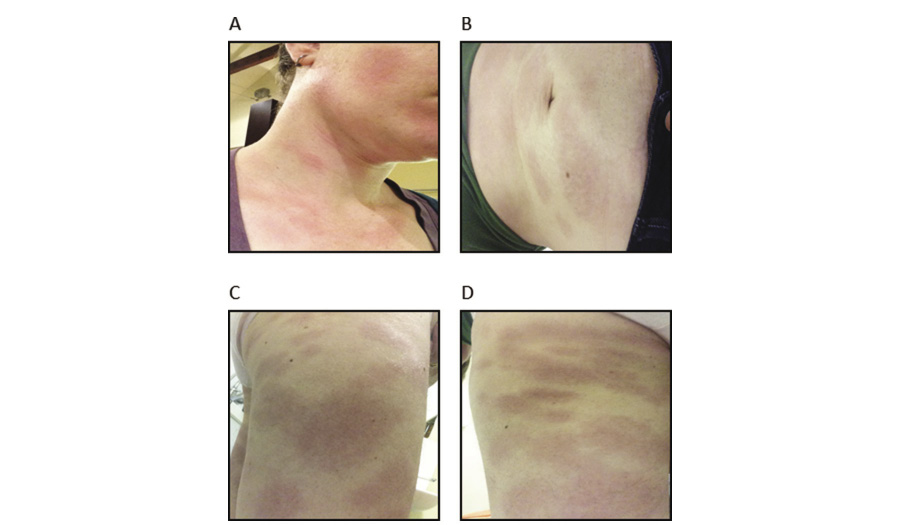
On presentation she was febrile and tachycardic. She appeared unwell with periorbital oedema, conjunctival suffusion and a mildly injected pharynx. She had shotty, subcentimetre anterior and posterior cervical lymphadenopathy and a palpable right epitrochlear lymph node. There was no organomegaly. Neurological examination was unremarkable, auscultation of the chest revealed vesicular breathing with no added sounds, and no cardiac murmurs were appreciated. There was a widespread, blanching, nonpruritic, macular rash with some confluence over the torso, limbs and face (Figure 1). Several lesions demonstrated central clearing with targetoid features. There was no mucosal involvement.
Figure 1 The patient presented with a widespread macular rash involving her (a) face and neck, (b) abdomen, (c) right leg and (d) left thigh. (a & d) Several lesions demonstrated central clearing with targetoid features
Initial laboratory results demonstrated normocytic anaemia (haemoglobin 90 g/l, normal range 115–160 g/l), neutrophilia (16.2 × 109/l, normal range 2.0–7.5 × 109/l), borderline lymphopenia (1.0 × 109/l, normal range 1.0–4.8 × 109/l), elevated C-reactive protein (279 mg/l, normal range <5 mg/l), coagulopathy (prothrombin time 18 s, normal range 11–13.5 s), thrombocytosis (546 × 109/l, normal range 130–400 × 109/l), normal renal function, normal creatine kinase and mildly deranged liver function (alkaline phosphatase 254 U/l, normal range 35–115 U/l; alanine aminotransferase 63 U/l, normal range 0–40 U/l; bilirubin normal). Blood film examination showed left shift and rouleaux; no parasites were visible. An electrocardiogram showed first-degree heart block.
The clinical impression was of a tick-borne illness and treatment was commenced with oral doxycycline 200 mg daily. Immunosuppressive medications were withheld. Over the following day she developed worsening neutrophilia and coagulopathy, and owing to concerns about dissemination and superadded bacterial infection, ceftriaxone 2 g once daily was added. Defervescence occurred 24 hours later accompanied by improving laboratory parameters and marked symptomatic improvement. Her rash and periorbital oedema resolved over the following 72 hours. The clinical diagnosis on discharge was early disseminated Lyme borreliosis.
There was no growth from blood, urine and throat swab cultures. A respiratory virus polymerase chain reaction panel, including Mycoplasma pneumoniae, was negative. Viral serologies were consistent with previous exposure to Epstein–Barr virus and toxoplasma. Serology for HIV, syphilis, hepatitis C virus, hepatitis B virus, cytomegalovirus IgM and parvovirus B19 IgM was negative. Acute and convalescent spotted fever and epidemic typhus group Rickettsia serology was negative. West Nile virus IgM was detected on both acute and convalescent samples with no IgG seroconversion and was felt to be a nonspecific reaction. Immunofluorescence for Anaplasma phagocytophilum IgG showed a nonspecific reaction.
Serum was referred to the National Lyme Borreliosis Testing Laboratory (NHS Highland) for further investigation. Enzyme immunoassay screening for B. burgdorferi IgM (B. burgdorferi IgM/IgG; Zeus Scientific, NJ, USA) was positive on the acute sample and seroconversion to IgG was confirmed by immunoblot (recomLine Lyme IgG, Mikrogen Diagnostik, Germany) on a convalescent sample, corroborating the clinical impression.
The patient completed 14 days of doxycycline and ceftriaxone as an outpatient. She was reviewed in clinic 3 weeks later and was systemically well with complete resolution of her rash. She described worsening arthralgia, requiring reintroduction of sulfasalazine and certolizumab, and she remains well in the community 12 months later.
Discussion
In this paper we present a case of early disseminated borreliosis in a patient receiving anti-TNF-α therapy for inflammatory arthritis. Certolizumab was discontinued during treatment and reintroduced 3 weeks later. Importantly, we document successful treatment with no relapse when TNF-α inhibition was reinitiated following standard duration antimicrobial chemotherapy.
Data from murine models suggest that TNF-α may have an important role in the immune response to Borrelia. In interferon-gamma (IFN-γ)-deficient mice, recombinant TNF-α is protective against destructive arthritis, enhances neutralising antibody production1 and prevents 95% of murine Borrelia infections when administered for 10 days after tick attachment.2 Additionally, spirochaete reactivation following anti-TNF-α treatment has been described in ceftriaxone-treated B. burgdorferi-infected mice, despite documented culture negativity post antimicrobial treatment.3
There is conflicting evidence in the literature as to the importance of TNF-α in the human immune response to Borrelia. In vitro stimulation of human peripheral blood mononuclear cells (PBMCs) by incubation with spirochaetes of B. burgdorferi results in dose-dependent production of TNF-α.4 Consistent with this study, TNF-α production was demonstrated after stimulation of PBMCs with B. burgdorferi lysate and the recombinant antigens OspA and OspC in a subsequent ex vivo study of humans with EM (n = 27), although IFN-γ production was greater.5 Additionally, increased numbers of Borrelia-induced TNF-α secreting dendritic cells have been identified in asymptomatic subjects (n = 7) compared with subjects with subacute neuroborreliosis (n = 7) and seronegative controls (n = 7), supporting a role for TNF-α in the early control of B. burgdorferi infection.6 However, a further study observed no difference in TNF-α serum levels in EM (n = 8), nor in serum or CSF levels in neuroborreliosis (n = 10), compared with controls (n = 7).7
The first steps of the immune response against B. burgdorferi occur in the skin, and this initial interaction may determine the outcome of infection. Skin biopsies from subjects with EM demonstrate mRNA expression of both pro- and anti-inflammatory cytokines, with IFN-γ and IL-10 most commonly identified. Macrophage-derived proinflammatory cytokines, including TNF-α, are also expressed.8 Pathway analysis of genes differentially regulated in EM biopsies (n = 18) relative to healthy controls (n = 11) has identified TNF-α as an important upstream regulator in early localised borreliosis, although interferon signalling remains the predominantly activated immune signalling pathway.9 In all cases, the sample sizes are too small for reliable conclusions. However, we hypothesise that iatrogenic TNF-α blockade could impair local innate immune control of Borrelia following initial inoculation into the skin, contributing to early haematogenous dissemination as seen in this case.
The optimum timing of reintroducing immunosuppressant medication following any infection is unknown and challenging questions were posed in this case based on data from the murine models. Reassurance was derived from the lack of a clearly demonstrated role for TNF-α in the human immune response to systemic Borrelia infection, coupled with the safe use of anti-TNF-α treatment for Lyme arthritis with no reported relapses,10 and the two clinical case reports detailed below.
The current case is the third report of borreliosis in a patient receiving anti-TNF-α therapy. The first report describes a case of EM (with no features of disseminated disease) in a 51-year-old female receiving methotrexate and etanercept for rheumatoid arthritis, who was treated with 300 mg doxycycline daily for 3 months.11 Methotrexate and etanercept were initially stopped but were reintroduced during therapy owing to polyarthritis and continued thereafter. In the second case, a 57-year-old female receiving adalimumab for psoriasis presented with Bannwarth’s syndrome (early disseminated neuroborreliosis).12 She was successfully treated with 14 days ceftriaxone and doxycycline, and was well 1 year later. Anti-TNF-α therapy was permanently discontinued. To the best of our knowledge, our case represents the first case of disseminated borreliosis in which TNF-α inhibition was successfully reinitiated following standard duration antimicrobial therapy.
Conclusion
We present the third case of Lyme borreliosis in an individual receiving anti-TNF-α therapy and provide reassurance that it is possible to successfully reinstitute such agents with no relapse of infection in the context of treated disseminated disease. This is relevant in the face of data from murine models suggesting an important role for TNF-α in the host defence against Borrelia, including the concerning finding of apparent spirochaete reactivation when TNF-α is inhibited after ceftriaxone treatment. Evidence for a critical role of TNF-α in the human immune response to systemic Borrelia infection is less compelling, although current data, supported by our case report, suggest that TNF-α may contribute to local innate control within the skin. As both the use of TNF-α inhibitors and the incidence of Lyme disease increases, the clinical situation we present may become more common, providing the opportunity to collect more data regarding TNF-α inhibition in Lyme borreliosis.
Acknowledgements
The authors would like to extend their thanks to the case patient for providing written, informed consent to use the clinical details and images from the case in this report. We would also like to thank Dr Roger Evans from the National Lyme Borreliosis Testing Laboratory (NHS Highland) and Professor David Dockrell (MRC/University of Edinburgh Centre for Inflammation Research) for their assistance in preparing the manuscript.
References
1 Christopherson JA, Munson EL, England DM et al. Destructive arthritis in vaccinated interferon gamma-deficient mice challenged with Borrelia burgdorferi: modulation by tumor necrosis factor alpha. Clin Diagn Lab Immunol 2003; 10: 44–52.
2 Zeidner N, Dreitz M, Belasco D et al. Suppression of acute Ixodes scapularis-induced Borrelia burgdorferi infection using tumor necrosis factor-alpha, interleukin-2 and interferon-gamma. J Infect Dis 1996; 173: 187–95.
3 Yrjanainen H, Hytonen J, Song XY et al. Anti-tumor necrosis factor-alpha treatment activates Borrelia burgdorferi spirochaetes 4 weeks after ceftriaxone treatment in C3H/He mice. J Infect Dis 2007; 195: 1489–96.
4 Defosse DL, Johnson RC. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun 1992; 60: 1109–13.
5 Glickstein L, Moore B, Bledsoe T et al. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect Immun 2003; 71: 6051–3.
6 Sjowall J, Carlsson A, Vaarala O et al. Innate immune responses in Lyme borreliosis: enhanced tumour necrosis factor-alpha and interleukin-12 in asymptomatic individuals in response to live spirochetes. Clin Exp Immunol 2005; 141: 89–98.
7 Widhe M, Grusell M, Ekerfelt C et al. Cytokines in Lyme borreliosis: lack of early tumour necrosis factor-α and transforming growth factor-α(1) responses are associated with chronic neuroborreliosis. Immunology 2002; 107: 46–55.
8 Mullegger RR, McHugh G, Ruthazer R et al. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J Invest Dermatol 2000; 115: 1115–23.
9 Marques A, Schwartz I, Wormser GP et al. Transcriptome assessment of erythema migrans skin lesions in patients with early Lyme disease reveals predominant interferon signalling. J Infect Dis 2017; 217: 158–67.
10 Steere A, Angelis S. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum 2006; 54: 3079–86.
11 Bonnet N, Guis S, Drancourt M et al. Borreliosis in a patient treated with anti-TNF alpha therapy: first case. J Eur Acad Dermatol Venereol 2011; 25: 367–8.
12 Merkak MI, Tomazic J, Strle F. Lyme neuroborreliosis in a patient treated with TNF-alpha inhibitor. Infection 2015; 43: 759–62.
